Hard tissue reconstruction in the oral and maxillofacial region due to trauma, tumor surgery or congenital deformities remains a challenge for reconstructive surgeons. The current preferred treatment includes the use of vascularized bone grafts, such as pedicle grafts or microvascular free grafts. This even extends to the use of computer-assisted modeling to preoperatively design the tumor resection and contour the microvascular free flap reconstruction by using a customized template. However, the current techniques have inherent disadvantages of donor site morbidity, risk of infection or non-acceptance of the flap as well as a prolonged healing and waiting time before dental rehabilitation can begin.
Bone regeneration is a well-organized but complex physiological process, in which different cell types and their activated signaling pathways are involved. In bone regeneration and remodeling processes, mesenchymal stem cells (MSCs) have a crucial role, during which their differentiation is regulated by specific signaling molecules (growth factors/cytokines and hormones) and their activated intracellular networks. Especially the utilization of this molecular machinery seems crucial to consider prior to developing bone substitute materials and cell-based constructs for bone regeneration.
Regenerative medicine is using the principles of “”tissue engineering”” and holds the promise for custom-tailored constructs with the potential to regenerate tissue in the host without significant donor-site morbidity and size limitation. This lecture aims to provide an overview of the current possibilities to optimize bone regeneration for cranio-facial reconstruction. First, the function and structure of bone will be discussed as well as the interaction between its main constituents with a specific focus on the mechanisms of bone mineralization. Second, the osteogenic potential of MSCs to become a key cellular resource for such regeneration and remodeling processes and the role of the scaffold material as such or as carrier for biofactors or stem cells will be addressed (Figure 1). Third, attention will be given to the main bio-inspired strategies to mineralize synthetic bone-substituting biomaterials.
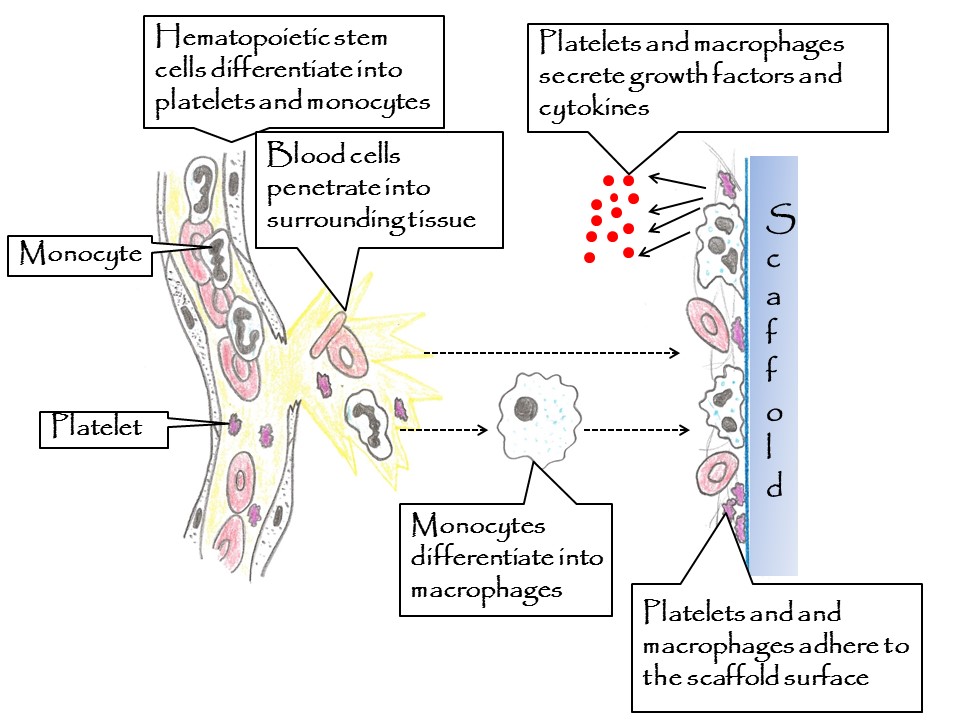
Figure 1. Cellular concept to explain the osteogenicity of a scaffold surfaces: Step 1: Blood cells penetrate into damaged body tissue (monocytes differentiate into macrophages), Step 2: Blood clot is formed on the scaffold surface (including blood cells and fibrin fibers), Step 3: Platelets and macrophages are activated and secrete growth factors and cytokines, which attract osteoprogenitor cells. The adhesion and activation of platelets and macrophages is controlled by the osteogenicc properties of the used scaffold material.
This work was supported by the Dutch Technology Foundation, Smartmix and the Royal Netherlands Academy of Science.; This work is also an AFIRM II project and was supported by the Army, Navy, NIH, Air Force, VA and Health Affairs to support the AFIRM II effort, under Award No. W81XWH-14-2-0004. The U.S.Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. Opinions, interpretations and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.