Introduction: To prevent adhesion formation after abdominal surgery, we have developed a thermally cross-linked gelatin film and previously reported its superior anti-adhesive effects with excellent peritoneal regeneration[1]. However, it may act as a scaffold convenient for tumor cell growth, thereby accelerating peritoneal dissemination when used in surgery for abdominal tumors since the gelatin film is made from a degenerated product of collagen which is the most abundant extracellular matrix protein in mammals and has been used as a scaffold in the field of regeneration medicine. In this study, we tried to clarify this issue in in vitro and in vivo experiments using mouse B16 melanoma cells and its carcinomatous peritonitis model, compared with HA/CMC film which is an anti-adhesive material composed of polysaccharides and has been used clinically.
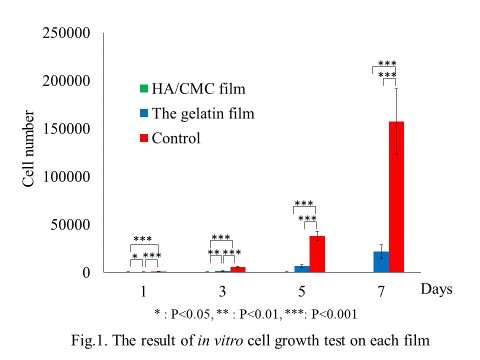
Materials, Methods and Results: At first, we examined the influence of the gelatin film for in vitro tumor cell growth. B16 melanoma cells were cultured on the gelatin film, HA/CMC film or without any film. Each viable cell number was counted with time. As the result, B16 melanoma cells grew on the gelatin film and HA/CMC film significantly lower than those in the control group.
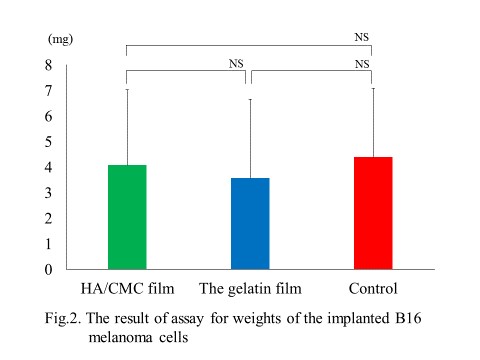
Next, we examined the influence of the gelatin film for peritoneal dissemination using B16 melanoma carcinomatous peritonitis model. After laparotomy, small parts of bilateral parietal peritoneum were removed mechanically. The injured site were covered with the gelatin film, HA/CMC film or without any films (control). Then, each mouse was inoculated intraperitoneally with the B16 melanoma cells, which has intrinsic black melanin pigmentation. At 7 days after the inoculation, the tumor weight of B16 melanoma at the injured sites were measured. The result showed no significant differences of the tumor weight among three groups.
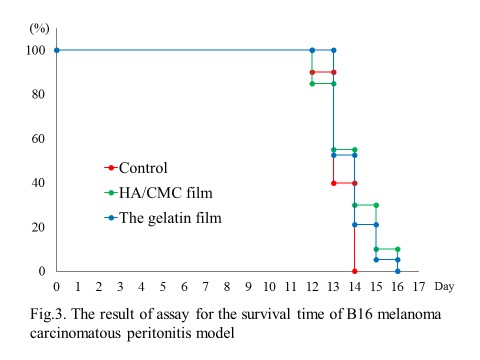
Furthermore, there was also no significant differences of the mean survival time of the mice among three groups with the gelatin film, HA/CMC film or without any films.
Discussion: In the in vitro experiment, the gelatin film as well as the HA/CMC inhibited the growth of B16 melanoma cells unexpectedly, compared with the control group. In the in vivo experiments using B16 melanoma carcinomatous peritonitis model, both films did not influence the tumor growth or the survival times of the mice inoculated with the tumors. These results suggests that the both types of films do not enhance the tumor growth at the injured sites at least.
Conclusions: Thermally cross-linked gelatin film does not act as a scaffold convenient for tumor cell growth, thereby accelerating peritoneal dissemination, when used in surgery for abdominal tumors.
References:
[1] H. Tsujimoto, A. Tanzawa, H. Miyamoto, T. Horii, M. Tsuji, A. Kawasumi, A. Tamura, Z. Wang, R. Abe, S. Tanaka, K. Yamanaka, M. Matoba, H. Torii, Y. Ozamoto, H. Takamori, S. Suzuki, S. Morita, Y. Ikada, A. Hagiwara, “Biological properties of a thermally crosslinked gelatin film as a novel anti-adhesive material: Relationship between the biological properties and the extent of thermal crosslinking”, Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2015; 103(7):1511-1518