Introduction: Type I collagen-gels, as scaffolds for vascular tissue engineering, have a high potential for supporting and guiding vascular cells in the regeneration process[1]. With this in mind, our project was to develop a set of easy-to-prepare collagen-based in vitro vascular wall models and experimental techniques to thoroughly characterize them.
Materials and Methods: Cellularized collagen gels were prepared by mixing vascular cells (106cells/ml) with a type I collagen solution (4g/L) as described before[2] and molded both in flat and tubular geometries. ECs were seeded on the surface of tubular constructs at the density of 8x105cells/cm2 using a homemade rotating bioreactor[3],[4]. Mono-culture (SMCs), bi-culture (SMCs and ECs) and tri-culture (FBs, SMCs and ECs) multilayered models were developed. The viscoelastic properties were studied on disk shaped constructs by compression test using MACH-1 (Biomomentum Inc), whereas on tubular constructs by stress-relaxation tests using ElectroPuls E1000 (Instron Corporation) longitudinally and circumferentially[4]. Protein expression by SMCs was investigated by western blot and the blood compatibility of constructs by clotting time assays. Tubular constructs were cultured in Instron Tissue Engineering & Regenerative Medicine (TERM) bioreactors with dynamic stimulations.
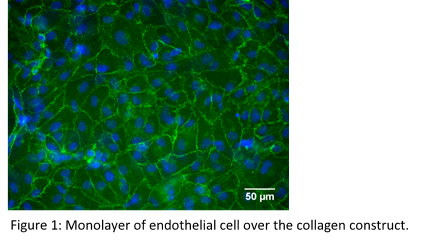
Results and Discussion: Mono-, bi- and tri-culture models of vascular wall composed of collagen and vascular cells in flat disk and tubular geometries were developed. The structure and cellular distribution of the tri-culture models closely matched those of native vascular wall, reproducing tunica intima, tunica media and tunica adventitia. Altogether, the models allowed to investigate the multiple intimate relationships existing among vascular cells. Importantly, SMCs and FBs remodeled the constructs by contracting the structure. A functional monolayer of ECs (Fig. 1) with anticoagulant properties was achieved.
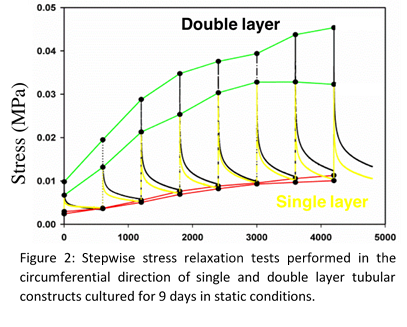
Viscoelastic properties were assessed through stepwise stress relaxation tests by stretching the sample at 10% strain, and maintained constant for 600s while monitoring the stress and the same procedure was repeated until 80% strain was achieved. It showed that the total stress (peak at the beginning of the relaxation, Fig. 2 green line) increased with the number of cellularised collagen layers, while the elastic properties (value at the end of relaxation, Fig. 2 red line) remained the same (Fig. 2). Anisotropic mechanical properties in longitudinal and circumferential directions were observed. Tubular constructs, strong enough to be transferred and cultured into the Instron-TERM bioreactor (Fig. 3) were obtained after 1 week static conditioning . Cyclic mechanical stimulations (0-50 mmHg, 1 Hz) allowed to further improve the mechanical properties of the constructs.
Conclusions: Herein, we engineered a platform of in vitro collagen gel-based vascular wall tissue models that can be used for physiological genomics studies. Sets of techniques were also developed to maturation and functional characterization of these structures biologically as well as mechanically. Furthermore, these models can serve as platforms for tightly controlled, high-content screening of drugs and devices in pharmacodynamic analyses.
This work was partially funded by NSERC-Canada, CIHR-Canada, FRQ-NT-Quebec, CFI-Canada. CL and DS were awarded of a doctoral scholarship from NSERC Create Program in Regenerative Medicine (www.ncprm.ulaval.ca).
References:
[1] Seifu D, et al., Nat Cardio. 2013
[2] Rajan N, et al., Nat Protoc. 2006
[3] Boccafoschi F, et al., Macromol Biosci. 2007
[4] Meghezi S, et al., JoVE. 2015