Type-1 collagen from rat-tail tendon has been widely used as scaffold in the field of tissue engineering. In the form of hydrogel, collagen serves as a matrix that mechanically and biologically supports three-dimensional cell growth and tissue regeneration. Considering their application as scaffold for small caliber engineered blood vessels (d<6 mm), the ability to withstand blood cyclic stress and compliance are crucial requirements. To address the elasticity in the collagen scaffold, we have explored the addition in the collagen hydrogel of byssus protein hydrolysate (BPH)[1] extracted from mussel byssal threads, which show remarkable combination of strength and extensibility.
In vitro self-assembly process leading to hydrogel gelation was optimized at pH 10 in 48-multiwell plates. The protocol of collagen gelation at pH 10 was adapted and modified from the work of Achilli et al[2]. Collagen buffer solutions with 101 mM of salt concentration (NaCl and NaOH) was used to solubilize the BPH, and mixed with the collagen solution to reach a final protein concentration of 2.8 mg/mL. The effect of temperature (4 and 22°C) on the gelation process was assessed by turbidimetric measurements. The resulting microstructure and matrix density were observed by scanning electron microscopy (SEM). The swelling ratio of the scaffold was also determined and compression-relaxation tests were carried to investigate the mechanical properties of the scaffold under compression.
Figure 1 shows the results of the turbidimetry measurements where the gelation time (t1/2) is defined as the required time to reach half of the final absorbance. The gelation time of collagen-BPH gel is longer (3.4 and 4.5 days) compared to the collagen control which took 4 and 13 hours at respective temperatures of 22°C and 4°C. Comparison of SEM images of collagen and collagen-BPH mixture at 22°C shows a denser matrix of collagen-BPH at the microstructural level (Figure 2). Moreover, collagen-BPH has a lower (58±5%) water uptake ratio than collagen (71±8%), suggesting greater chemical interactions within the gel leading to a more compact matrix. Compression test shows that BPH incorporation increases scaffold relaxation time, which corresponds to the higher residual force. The compressive and tensile modulus also increases by 12±4% compared to collagen control at pH 10. In conclusion, the addition of BPH to collagen hydrogel increases the density and strength of the scaffold, and shows great potential to improve the collagen matrix elasticity. Further work is required to assess the biological properties of this new scaffold.
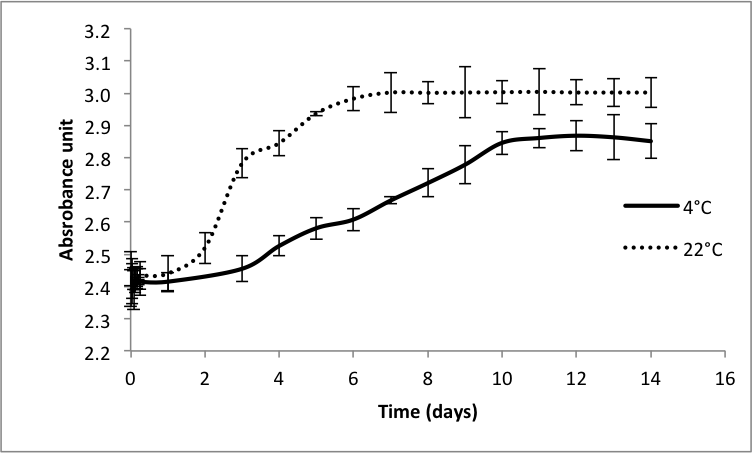
Figure 1. Turbidity measurements for collagen-BPH gels prepared at pH 10 at 4 °C and 22 °C
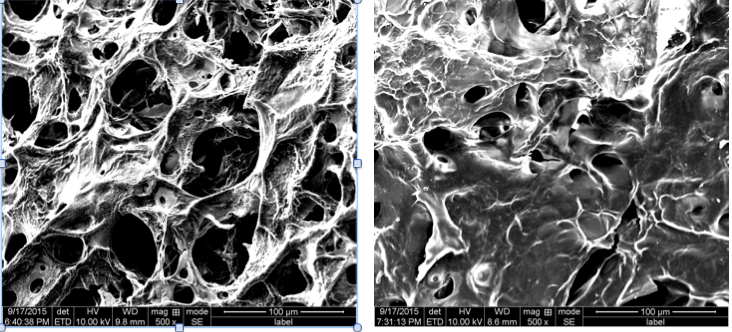
Figure 2. SEM images of collagen control (left) and collagen-BPH (right) samples prepared at pH 10 and 22°C
This work was partially funded by NSERC-Canada, FRQ-NT-Quebec, CFI-Canada, and the Centre Québécois sur les Matériaux Fonctionnels (CMQF).
References:
[1] Byette, F., Pellerin, C., Marcotte, I. J. Materials Chemistry B 2, 6378–6386 (2014)
[2] Achilli, M., and Mantovani, D., Polymers 2, 664-680 (2010)