Introduction: Stem cell therapy offers a promising approach to regenerate damaged cardiac tissue after acute myocardial infarction. Recent evidences have attributed such improvement to the broad repertoire of angiogenic paracrine factors (growth factors, chemokines and exosomes) secreted by the stem cells, known as secretome[1]. Here, we intend (i) to efficiently bio-manufacture cell secretome from 3D stem cell aggregates using a microfluidic system and (ii) efficiently deliver the harnessed secretome for cardiac repair and revascularization applications using a hydrogel-based injectable delivery system.
Experiments and Results: As a first step, a microfluidic device consisting of deep concave microwell arrays was fabricated using soft lithography microscale techniques. Results demonstrate that bone marrow derived human mesenchymal stem cell (hMSC) suspensions, injected to the microfluidic chip through inlet port, form uniformly sized aggregates (~220μm diameter) within 2 days. Computational analysis confirmed rapid oxygen diffusion in the microwells. 3 days post-incubation, hMSC secretome (4.5-9 times increase in angiogenic protein concentrations compared to 2D culture including VEGF, FGF, Angiogenin) was collected, leaving behind the hMSC aggregates at the bottom of the well.
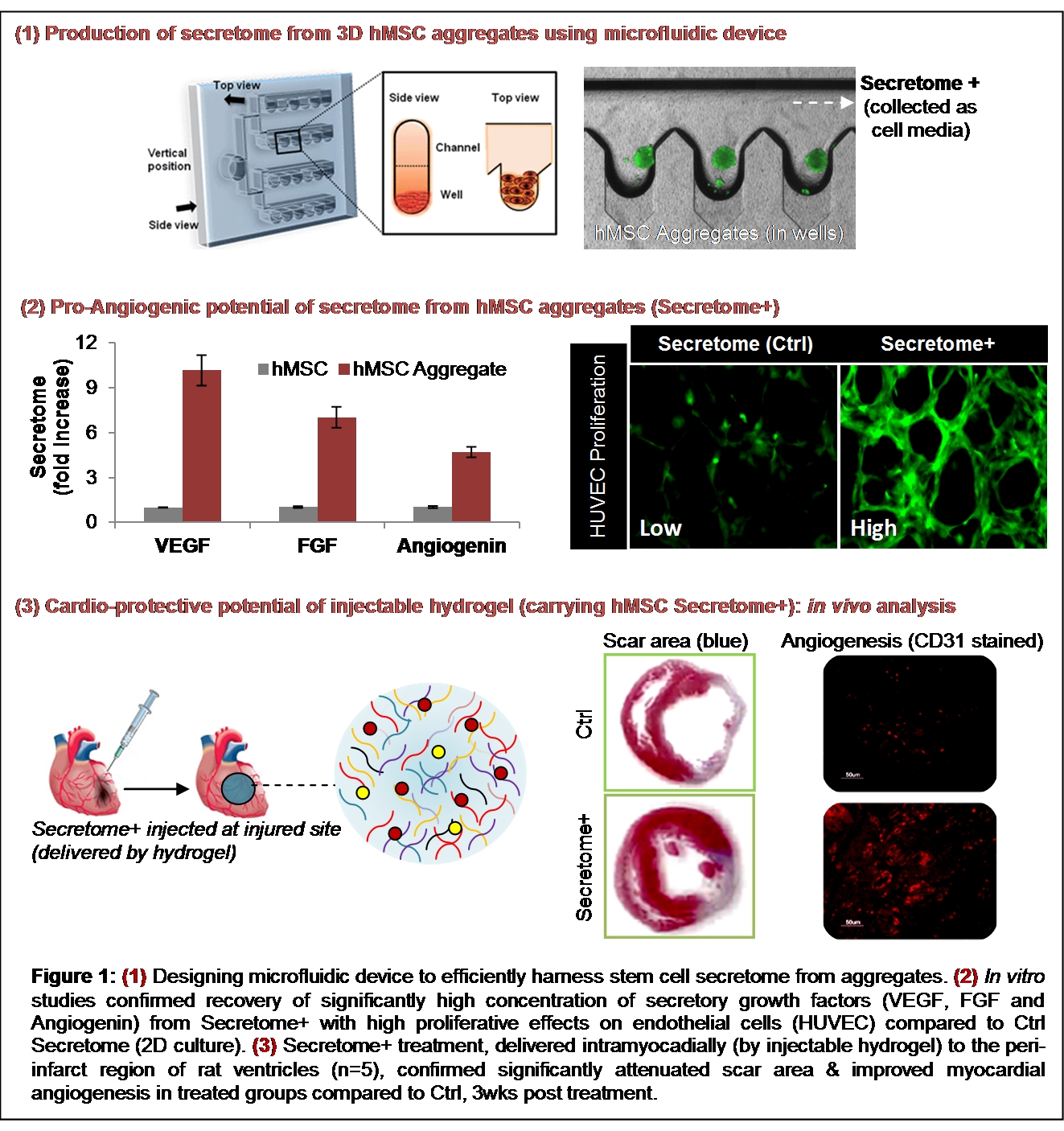
As a next step, a physically cross-linked injectable nanocomposite hydrogel, comprising of silicate nanoplatelets and gelatin, was formulated to deliver the harnessed hMSC secretome to the target site. In vitro studies confirmed controlled release of bioactive hMSC growth factors from the hydrogel which induced proliferation of human endothelial cells (HUVEC). For in vivo studies rat model with acute myocardial infarction (n=5) was used. The secretome carrying hydrogel was injected intramyocardially at the per-infarct region of the heart, post infarction as mentioned in earlier work[2]. After 3 weeks, the animals were sacrificed and the injected tissue regions were examined for histological analysis. Masson’s Trichome staining confirmed reduced scar area in the treated Secretome+ group (3D cell aggregates) compared to control group (secretome from 2D cell culture). Immunostaining with CD31 antibodies also confirmed significantly high local myocardial angiogenesis at the injected site in Secretome+ group. These data confirms the angiogenic therapeutic potential of the developed hydrogel in vitro and in vivo.
Conclusion: Take together, this study reports a novel strategy to utilize cell secretome, as an alternate to traditional cell therapy, for myocardial regeneration therapy. The strategy can also be used for other biomedical applications, such as wound healing and vascular tissue engineering.
Arghya Paul likes to acknowledge the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences, National Institutes of Health (NIH), under Award Number P20GM103638.
References:
[1] Burdon TJ, Paul A, Noiseux N, Prakash S, Shum-Tim D (2011). Bone marrow stem cell derived paracrine factors for regenerative medicine: current perspectives and therapeutic potential. Bone Marrow Res. 2011:207326.
[2] Paul A et al (2014). Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano; 8(8):8050-62.