Introduction: Crystalline Mg alloys specifically designed for biomedical applications are currently being developed and investigated to address the rapid degradation of current Mg-based biomaterials, therefore meeting clinical requirements. The objective of this study was to evaluate the in vitro degradation rates of binary Mg-xSr and ternary Mg-1Ca-xSr alloys, and determine viability of bone marrow derived mesenchymal stem cells (BMSCs) at the cell-biomaterial interface through the direct culture method[1].
Materials and Methods: Mg-xSr and Mg-1Ca-xSr (x = 0, 0.2, 0.5, 1, 2 wt. %) and non-culture-treated glass slides were investigated in this study. Rat BMSCs were harvested and cultured according to details found herein[1]. BMSCs (P2) were seeded directly onto the sample surfaces at 4x104 cells/cm2 and incubated in DMEM + 10% FBS + 1% penicillin/ streptomycin under standard cell culture conditions for 72 hr. The cell culture media was collected and replenished at 24 hr intervals and Mg2+ ion concentrations were measured with an inductive coupled plasma optical emission spectrometer (ICP-OES) to determine the degradation rate (calculated as mass loss per unit area per day). After incubation, samples were fixed with 4% formaldehyde and stained with 4’,6-diamidino-2-phenylindole dilactate (DAPI) nucleic acid stain and Alexa Flour® 488-phalloidin cytoskeleton stain. DAPI-stained nuclei were observed using a fluorescent microscope and counted per unit area to determine cell density. Cell densities directly on the surface of each sample (ρi) were normalized by the cell density of the positive control, i.e. cells only on tissue culture polystyrene (ρBMSC).
Results and Discussion: The mean degradation rate of the binary Mg-xSr alloys was generally lower compared with the ternary Mg-1Ca-xSr alloys, Mg control, and Mg-1Ca-0Sr (Fig. 1a). In the binary and ternary compositions, the alloys with 2 wt. % Sr generally showed the lowest degradation rate. Additionally, when evaluating BMSC adhesion and viability at the cell-biomaterial interface (Fig. 1b), our results showed that the ternary Mg-1Ca-xSr alloys generally showed a higher amount of adhered and viable BMSCs compared with their binary alloy counterparts, Mg control, and Mg-1Ca-0Sr alloy (Fig. 1c).
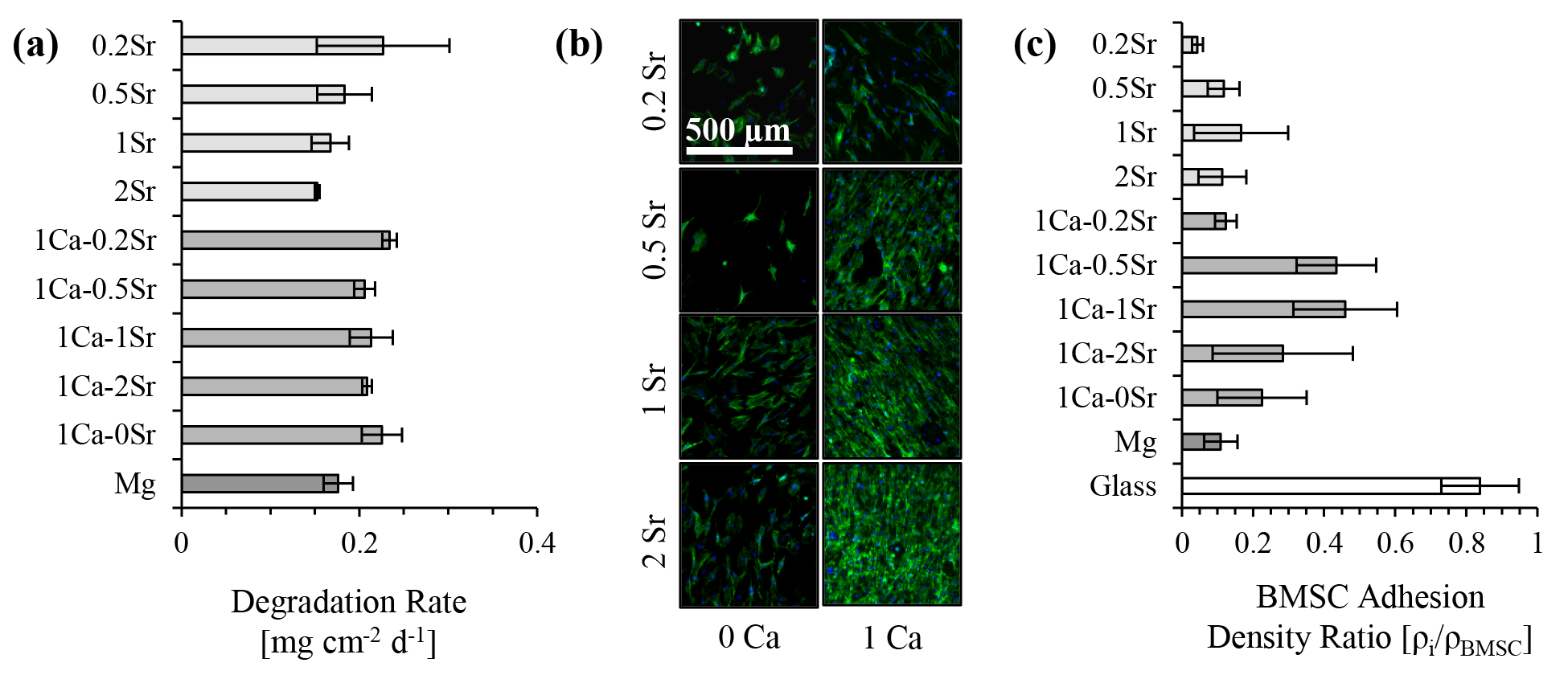
Conclusion: These results suggest that implant-tissue integration of Mg-based biomaterials for orthopedic applications could be improved by the inclusion of both Ca and Sr as an alloying elements; however, at the cost of slightly faster degradation rates compared with their binary counterparts. Degradation rate and cell viability analyses indicate that Mg-1Ca-0.5Sr, Mg-1Ca-1Sr, and Mg-1Ca-2Sr alloys should be further investigated for orthopedic implant applications.
UCR MSRIP program for supporting Marisa Lopez and Mayra Cortez; CIRM Bridge for supporting Amy Sallee
References:
[1] Cipriano AF. Acta Biomater. 2015 12:298-321