Introduction:In dental practice, polymeric membranes are used as a barrier to prevent soft tissue ingrowth and create space for slowly regenerating periodontal and bony tissues. This concept is known as guided tissue regeneration (GTR) or guided bone regeneration (GBR). Although present polymeric products show positive results in clinical studies, their weak mechanical properties and poor bone regeneration capacity are still major challenges. To overcome these problems, recent research efforts focus on the incorporation of osteoconductive materials into the membranes. The aim of this study was to develop a bioactive and biodegradable GTR/GBR membrane based on silica-and poly(ε-caprolactone) (PCL) nanocomposite fibers prepared by electrospinning..
Materials and Methods: Silica nanoparticles (NPs) with an average diameter of 80 nm were prepared by the Stober method[1]. PCL-based membranes containing different amounts of silica NPs (0, 25, 50 and 75 wt.%) were prepared by electrospinning, being named respectively S0, S25, S50 and S75. The morphology and structure of the membranes were inspected by scanning electron microscope (SEM), transmission electron microscope (TEM), X-ray diffraction (XRD) and attenuated total reflectance infrared spectroscopy (ATR-FTIR). The mechanical performance of membranes was evaluated by a tensile test. Simulated body fluid (SBF) immersion tests and cell culture studies using osteoblast-like MC3T3-E1 cells were performed to evaluate the in vitro biological performance of the membranes.
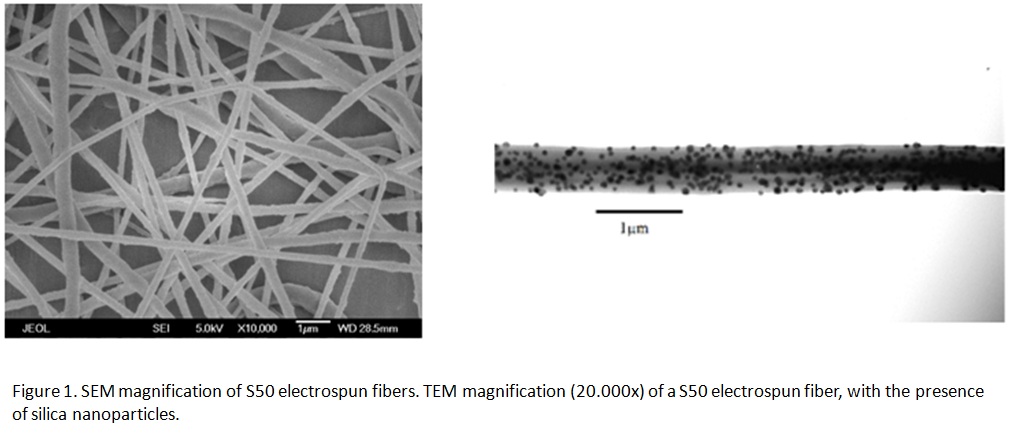
Results and Discussion: Nanocomposite membranes with average fiber diameter ranging from 0.1 to 1 µm could be produced by electrospinning. Remarkably, we were able to produce mechanically robust nanocomposite fibers with silica contents up to 75wt.%. Incorporation of silica NPs in the PCL-matrix led to the decrease of the fiber diameter. TEM evaluations revealed that silica NPs were located in the interior of the electrospun fibers.
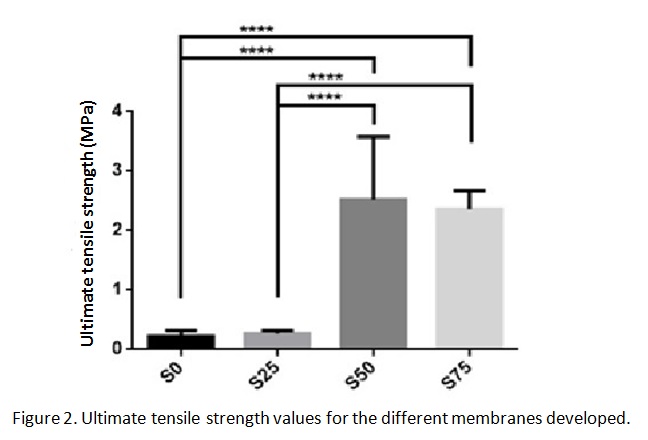
The tensile test showed a significant increase in the ultimate tensile strength for the samples with 50% and 75% of silica content, indicating a reinforcing effect of the silica NPs.
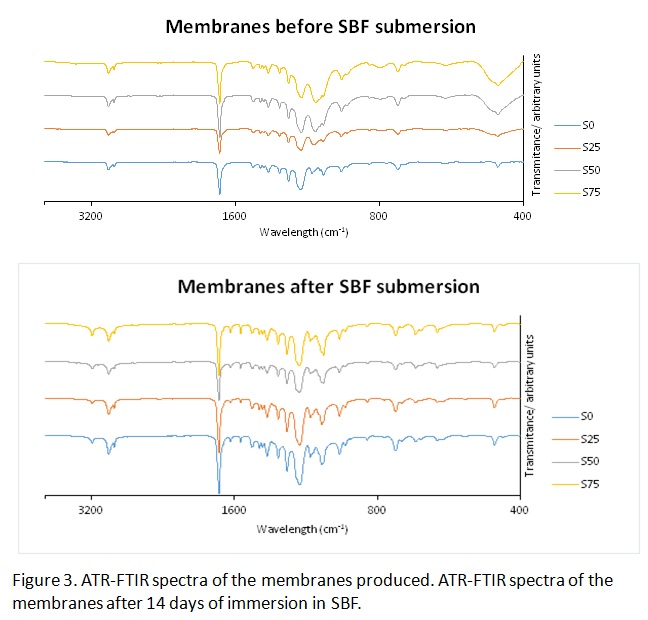
Interestingly ATR-FTIR spectra of the membranes containing silica NPs immersed in SBF solution have shown the presence of bands that can be assigned to CO32- groups (1554 cm-1 and 1631 cm-1) and PO43- groups (600 cm-1 and 664 cm-1) in apatite minerals[2].
The in vitro cell culture tests showed that the materials prepared did not present any cytotoxic effect.
Conclusion: Nanocomposite electrospun membranes could be successfully produced with up 75wt.% of silica content. Incorporation of the silica NPs in the membranes’ composition presented an enhancement in their mechanical strength and hydroxyapatite formation ability, and supported osteoblast-like cell proliferation. The membrane system can be used as a prototype for the further development of an optimal membrane for clinical use.
Netherlands Institute for Regenerative Medicine (NIRM, grant No. FES0908); AgentschapNL (IOP Self Healing Materials, Project no. SHM012014).; Evert Wijlemans
References:
[1] Ismail A.M. Ibrahim, A. A. F. Z., Mohamed A. Sharaf (2010). "Preparation of spherical silica nanoparticles: Stober silica." Journal of American Science 6(11): 985-989.
[2] Sadjadi, M. S., et al. (2011). "Silica enhanced formation of hydroxyapatite nanocrystals in simulated body fluid (SBF) at 37 degrees C." Materials Chemistry and Physics 130(1-2): 67-71.