Introduction: Idiopathic pulmonary fibrosis (IPF) is a chronic and fatal disease characterized by progressive stiffening of the lung matrix[1]. IPF is perpetuated by pathologically activated fibroblasts that are significantly more contractile than normal lung fibroblasts (NLFs)[2], however the underlying mechanisms of IPF remain poorly understood. Our objective in this work is to quantify the dynamics of healthy and diseased primary fibroblast cell-matrix interactions in physiologically-realistic, but precisely-defined culture systems. Understanding the biomechanics underlying IPF disease progression will provide insight to potential drug targets and therapies.
Materials and Methods: A collagen microgel contraction assay was previously developed to rapidly measure cell contractile forces[3] (Fig 1A). The micro-scale platform circumvents transport limitations inherent in conventional bulk culture systems, enabling direct comparisons between cell types. An aqueous two-phase system consisting of cells/collagen/dextran phase and a polyethylene glycol phase is used to print a microgel in a cell culture environment. Remodelling of the microgels by normal and IPF patient-derived primary fibroblasts cells is monitored optically to measure cellular contraction. Collagen contraction experiments were followed with single-cell traction force microscopy (TFM) using standard techniques[4].
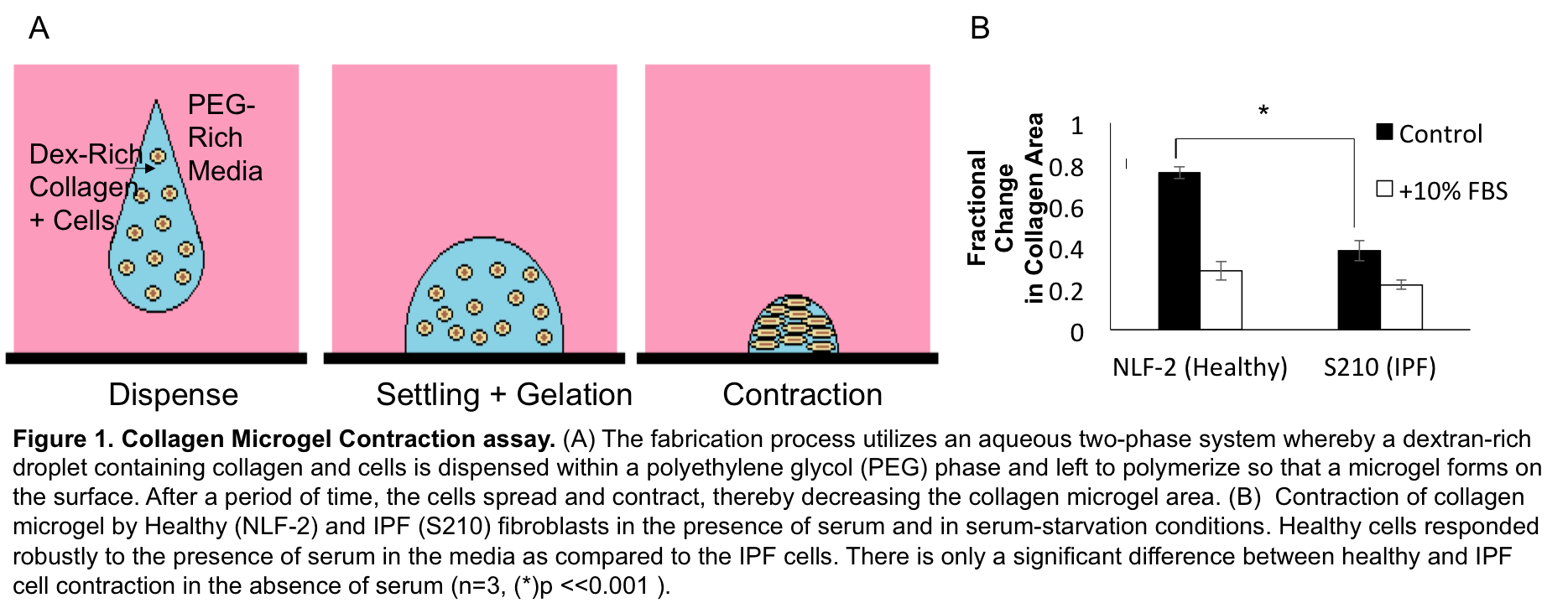
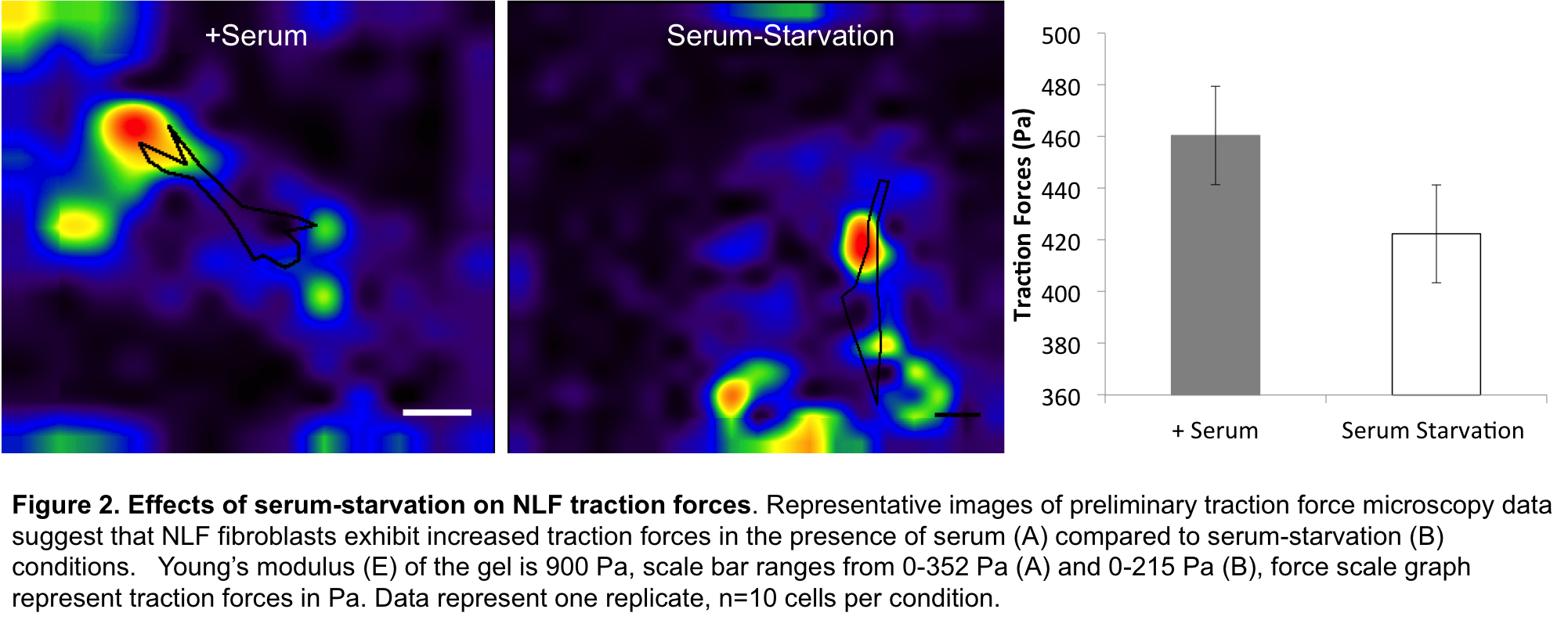
Results: The collagen contraction assay indicated that NLF and IPF cells were indistinguishable in terms of contractile remodelling in the presence of fetal bovine serum (FBS). In contrast, serum-free culture showed marked increases in contractile activity for IPF patient samples (Fig1B). Since NLFs are FBS-sensitive and IPF contraction levels remain consistently high, this suggests that high contractility is a pathological baseline state in IPF. Furthermore, since serum abrogates these differences, and IPF cells are inherently more contractile than healthy cells[1],[5], this suggests that serum-supplemented cultures may not provide an adequate platform to study IPF. Although collagen contraction assays are fast and simple, collagen structure and geometry can be unstable providing only a rough estimate of cell forces[6]. Thus we confirmed the results obtained by measuring forces at the cellular level using TFM. Preliminary analysis suggests that NLFs exert greater traction forces in the presence of serum compared to serum-starvation conditions (Fig2), and that IPF cell traction forces were not affected by serum (data not shown).
Conclusion: The results suggest that IPF fibroblast dynamics are different, and distinctly regulated from healthy lung fibroblasts. Moreover, there appears to be no functional difference between healthy and IPF dynamics in regularly supplemented serum. Future experimentation will explore the effects of various soluble factors on healthy and IPF dynamics in order to further model microscale IPF physiology.
This work supported by a Natural Sciences and Engineering Research Council of Canada Discovery Grant and Undergraduate Student Research Award, and a Province of Quebec Fonds Nature et Technologies New Investigator award.
References:
[1] Marinković, A., Mih, J. D., Park, J. A., Liu, F. & Tschumperlin, D. J. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness. American Journal of Physiology - Lung Cellular and Molecular Physiology 303, L169-180, doi:10.1152/ajplung.00108.2012 (2012).
[2] Marinkovic, A., Liu, F. & Tschumperlin, D. J. Matrices of physiologic stiffness potently inactivate idiopathic pulmonary fibrosis fibroblasts. American journal of respiratory cell and molecular biology 48, 422-430, doi:10.1165/rcmb.2012-0335OC (2013).
[3] Moraes, C., Simon, A. B., Putnam, A. J. & Takayama, S. Aqueous two-phase printing of cell-containing contractile collagen microgels. Biomaterials 34, 9623-9631, doi:10.1016/j.biomaterials.2013.08.046 (2013).
[4] Style, R. W. et al. Traction force microscopy in physics and biology. Soft matter 10, 4047-4055, doi:10.1039/c4sm00264d (2014).
[5] Parker, M. W. et al. Fibrotic extracellular matrix activates a profibrotic positive feedback loop. The Journal of clinical investigation 124, 1622-1635, doi:10.1172/jci71386 (2014).
[6] Li, B. & Wang, J. H. C. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. Journal of Tissue Viability 20, 108-120, doi:http://dx.doi.org/10.1016/j.jtv.2009.11.004 (2011).