Introduction: Avoiding biofilm and blood clot formation are major issues in the field of implantable medical devices. When the devices are implanted into the body, the host tissues react to the surface of these foreign materials, and cause the foreign body reaction along with inflammatory reactions, which thus lead to device failure[1].
To overcome these issues, surface modification of the device is required to increase the biocompatibility and evade the unwanted biological processes. Up to date, It have been reported that hydrated surface exhibited nonfouling property and various kinds of hydrophilic materials were found to be effective for surface hydration. Many studies also suggested that zwitterionic molecules have greater antifouling effect than hydrophilic molecules due to their strong electrostatically induced hydration effect. We previsously reported a facile surface modification method using tyrosinase-catalyzed oxidative reaction. Tyrosinase is an enzyme that catalyzes the oxidation of phenol molecules into catechol and o-quinone groups having adhesive property[2].
Through this simple technique, we can immobilize bioactive molecules including phenol moieties onto any kind of substrates to enhanced bioactivities of the substrates. Herein, we report a facile immobilization method of zwitterionic sulfobetaine polymer (Poly(sulfobetaine-co-tyramine), pSBTA) onto PU substrates for improving antifouling activity.
Materials and Methods: Tyrosinase-reactive pSBTA was synthesized by radical co-polymerization of sulfobetaine methacrylate (SBMA) and methacrylic acid (MAA). Next, tyramine (TA) molecules were conjugated to the co-polymers through EDC/NHS chemistry. The chemical structure of pSBTA was characterized by 1H NMR and the amount of phenol moiety in polymer was measured by UV spectrometer. Using this functionalized polymers, we utilized a rapid grafting tools by a simple deeping method in mild conditions (pH 7.4 buffer, 37℃) using tyrosinase-catalyzed reactions (Fig. 1). In order to characterize the functionalized polyurethane surfaces, static water contact angle measurement and X-ray photoelectron spectroscopy (XPS) were performed, and in vitro fibrinogen absorption was investigated.
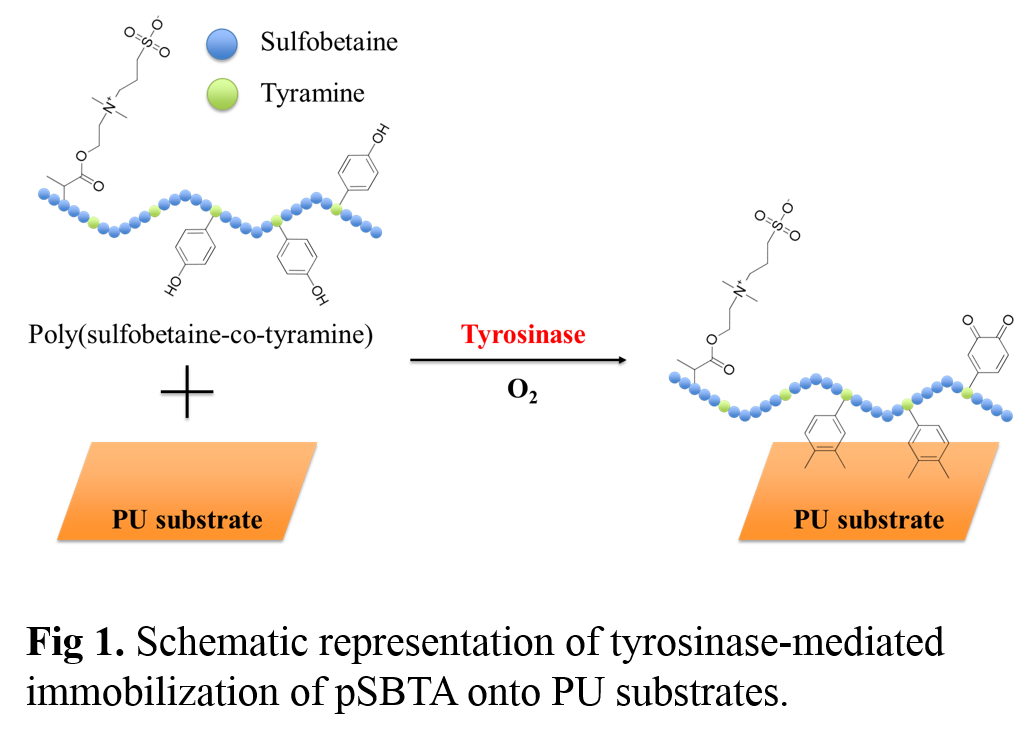
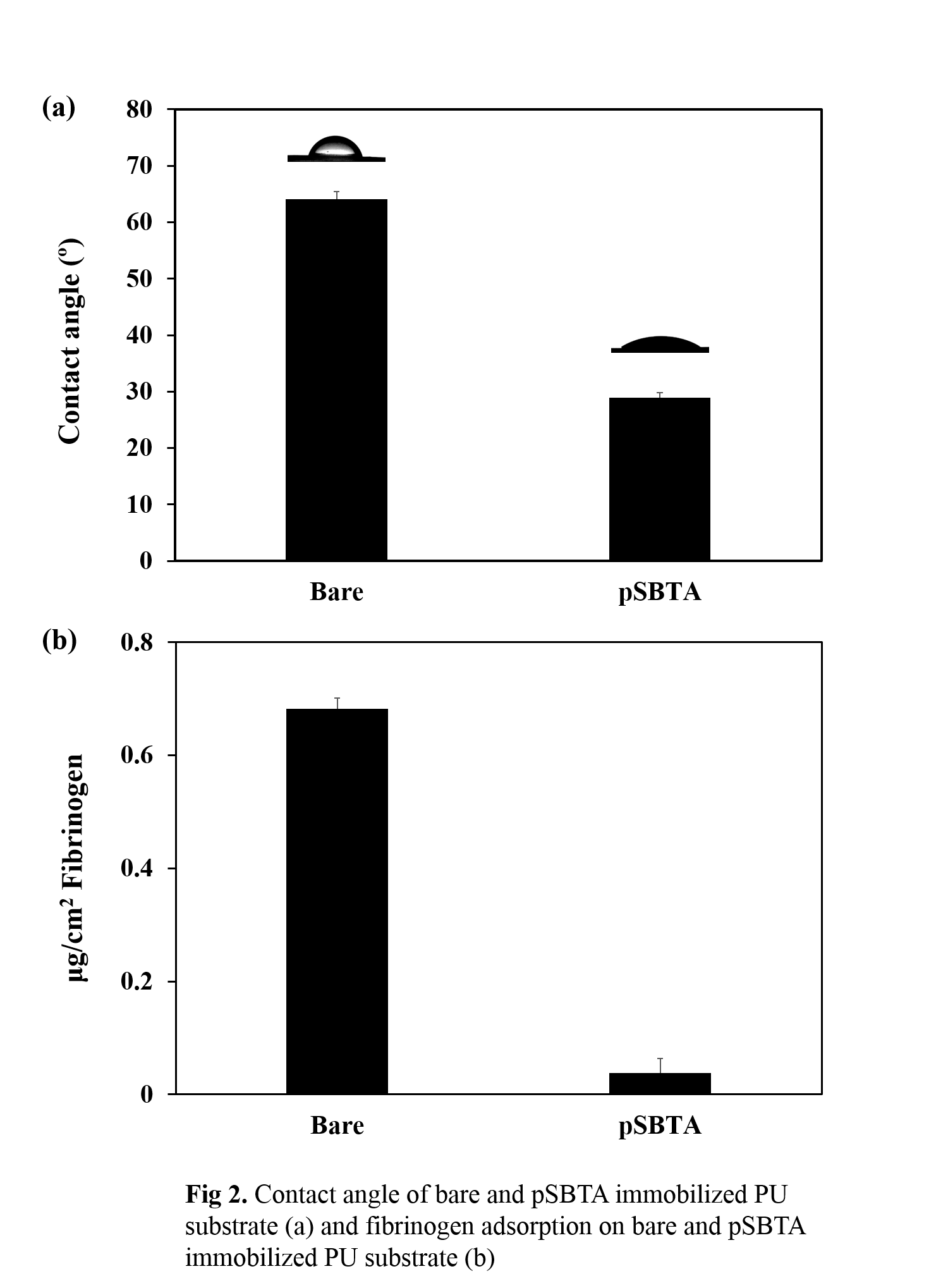
Results and Discussion: The 1H NMR spectra clearly showed the characteristic peaks corresponding to phenol groups of pSBTA polymer, which appear at 6.8-7.1 ppm, demonstrating tyramine molecules were sucessfully conjugated to poly(sulfobetaine- co-methacrylic acid) co-polymer. In addition, The amount of phenol moiety (47μmol/g of polymer) in pSBTA polymer, was measured by UV spectrometer (275nm). In order to evaluate the functionalized polyurethane surfaces, static water contact angle was measured, showing that contact angle of the modified surface was significantly decreased compared to unmodified polyurethane substrate (64 to 29 degree, Figure 2a) due to the hydrophilic sulfobetaine polymer. In addition, we evaluated antifouling effect of the modified surfaces through fibrinogen absorption study. The result showed that amount of adsorbed fibrinogen was decreased about 94% (0.68μg/cm2 to 0.04μg/cm2) in modified surface compared to unmodified surface (Figure 2b).
Conclusions: We developed a facile method for simple surface grafting of zwitterionic sulfobetaine polymer onto polyurethane surface via tyrosinase-catalyzed reactions. The results indicate that the surface modification tools have a great potential to make a highly hydrated surface, which leads to design an anticoagulant and antibacterial surfaces of medical devices.
This work was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) grant funded by the Ministry of Science, ICT & Future Planning(NRF-2015M3A9E2028577)
References:
[1] Harding, et al, Trends Biotechnol., 2014, 32(3), 140-146
[2] Y. Lee, et al., Colloids Surf., B., 2013, 102, 585-589