Introduction: In the past two decades, researchers have focused on developing new polymers for drug delivery contact lenses to obtain extended and controlled drug release. However, the release properties of these new materials are characterized using models that do not consider the limited tear volume and the constant tear replenishment in the front of the eye. This study investigated the release of two surrogate drugs from contact lenses using an in vitro cell and a dynamic drug release models. Results were compared to a fixed volume release model.
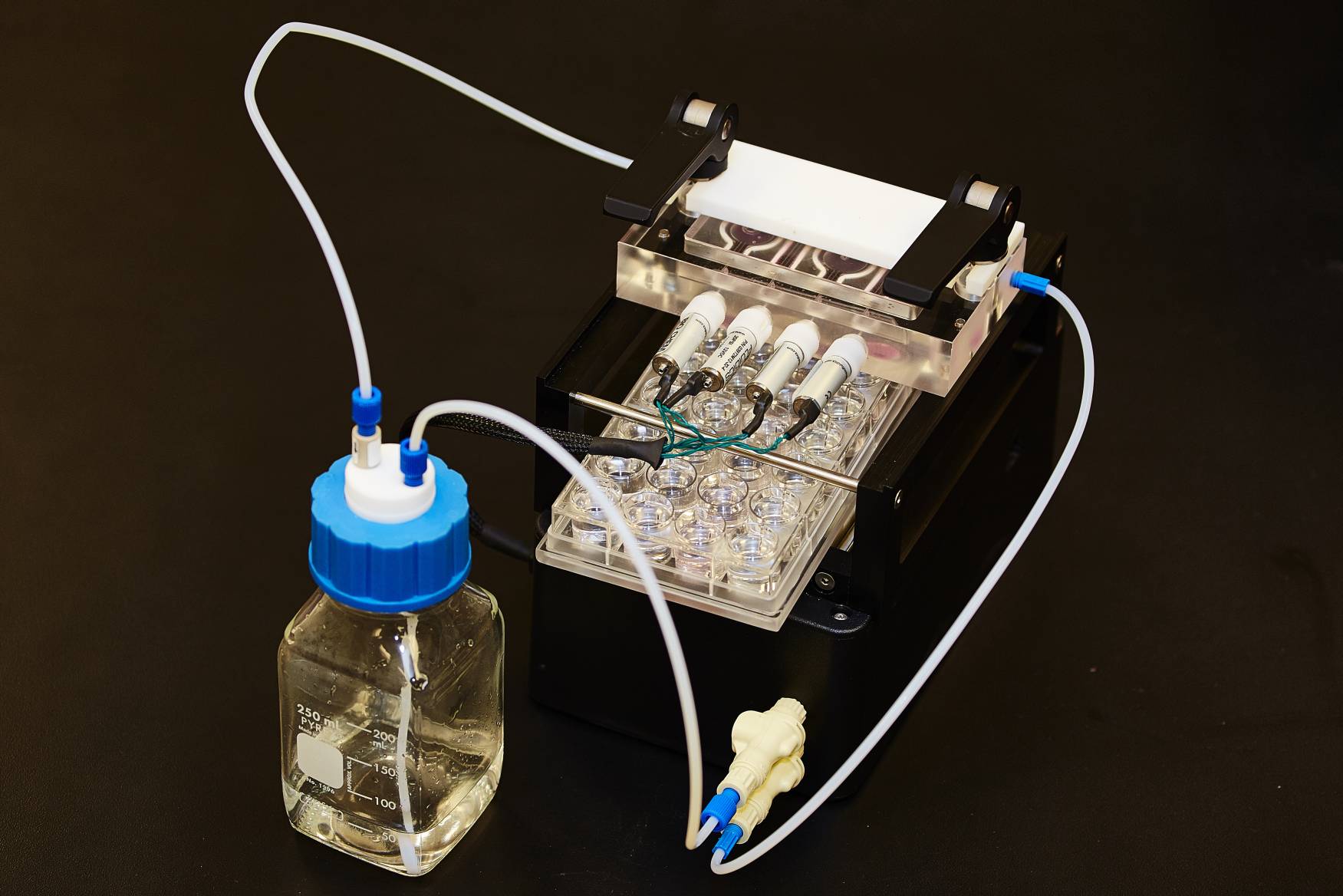
Materials of Methods: The in vitro cell model used diffusion through a monolayer of human corneal epithelial cells grown on a polyethylene terephthalate filter. The dynamic drug release model, shown below, was developed to simulate the physiological conditions of the front of the eye. Tear replenishment was modeled by an intermittent flow (5 times per minute to mimic blinking) of phosphate buffer saline (PBS) at an average rate of 20μL/min. The fixed volume release was conducted in 1.5mL of PBS. Conventional and silicone hydrogel contact lens materials (balafilcon A; senofilcon A; etafilcon A; hilafilcon B; polymacon) were soaked in 1.5mL of fluorescein sodium (surrogate hydrophilic drug) and rhodamine B (surrogate hydrophobic drug) for 24hrs. Release from lenses was measured for 48 hours in the cell and fixed volume models and for 24 hours in the dynamic drug release model (DDRM).
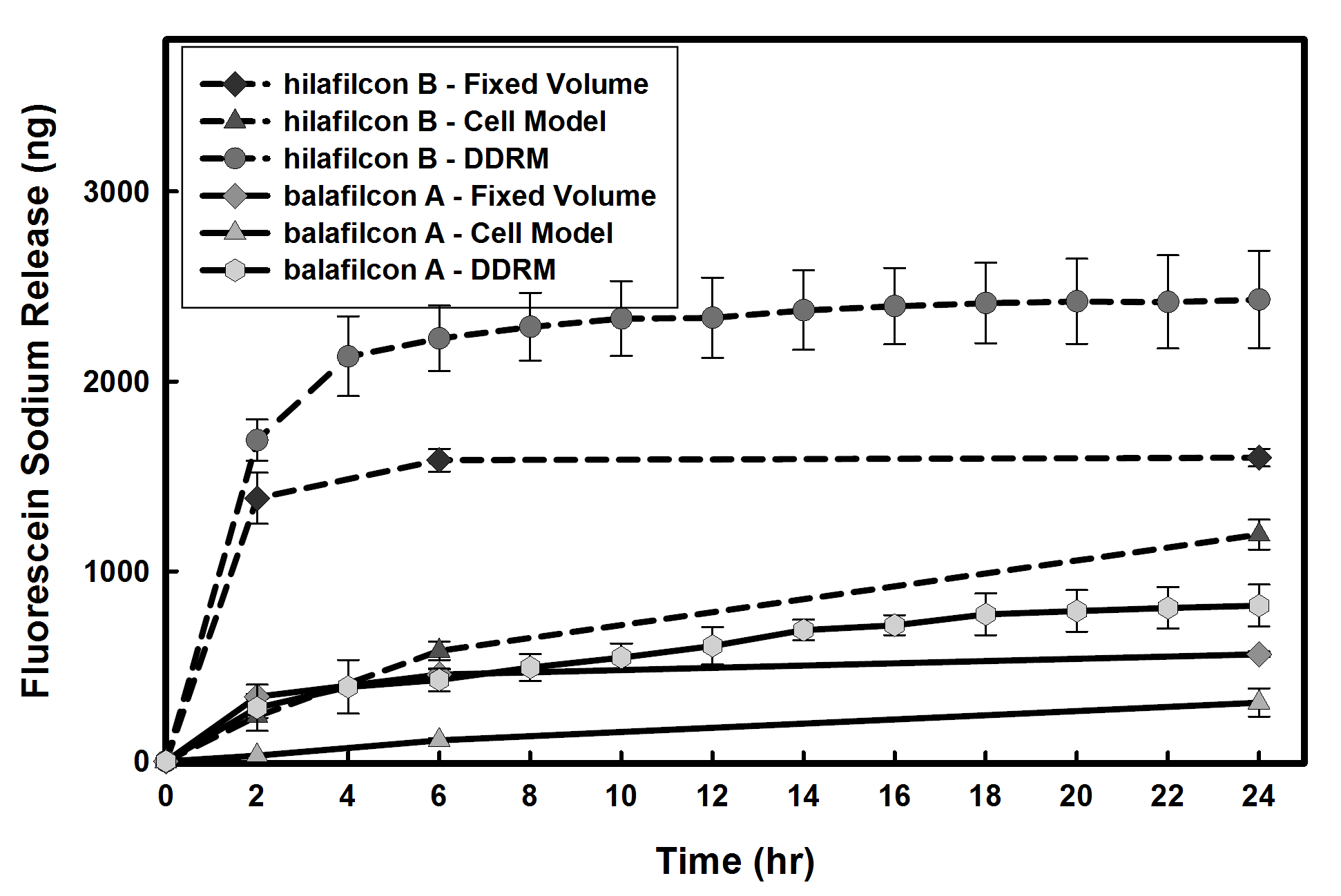
Results and Discussion: In the fixed volume model, following an initial burst, release from all contact lens materials plateaued after 6 hours. Release of fluorescein sodium from conventional and silicone hydrogel contact lens materials through the in vitro cell model was significantly slower (p<0.005) with an extended release up to 48 hours. This is likely due to the cell layer acting as a barrier against drug diffusion.
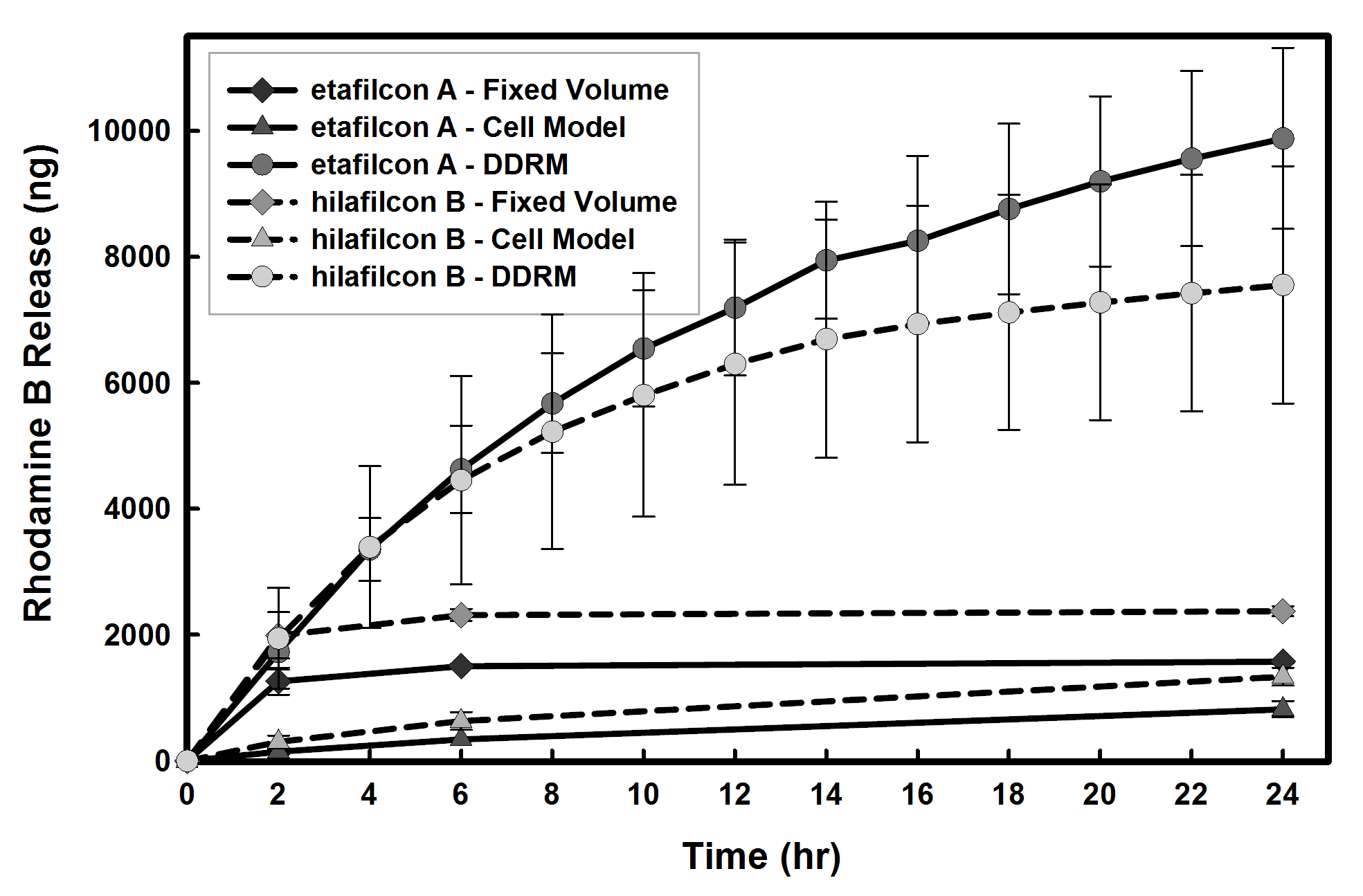
The release of rhodamine B from balafilcon A and senofilcon A (both silicone hydrogels) in the cell model reached ~1μg compared to ~3μg in the fixed volume release model. In the DDRM, the amount of rhodamine B release from both silicone and non-silicone hydrogels increased 2 to 6 times compared to the fixed volume model. For etafilcon A, more than 80% of available drug was released compared to 13% in the fixed volume model.
Conclusion: Our results highlight the importance of selecting appropriate in vitro experimental models to predict the release profile and total amount of released drug under physiological conditions for ocular drug delivery systems. Where the conventional models may predict a limited release of hydrophobic compounds from contact lens materials, a dynamic model will provide evidence of a significantly different release rate. A dynamic release model represents a valuable cell-free and cost effective solution to identify promising candidates for future in vivo studies.