Introduction: Biopolymer hydrogel scaffolds are widely used in tissue engineering as they have some physicochemical similarities to extracellular matrix (ECM). A crucial difference, however, is that native ECM is anisotropic in both material and cellular composition. As a result any mechanism for integrating multiple materials and cell types into a single construct is of great interest as it would facilitate the creation of a more native environment in vitro. 3D bioprinting is one such technique that has showed promise as a potential a mechanism for producing integrated tissues. Bioprinting also has the potential to create tissue engineering constructs of defined shape with detailed complexity which could be applied to fabricate implants for patient-specific defects or replicating interfaces between different tissues such as bone and cartilage. Limitations in bioprinting however, are often caused by the small range of biomaterials that are compatible with the technique and that have mechanical properties that resemble native ECM. Here we present a technique that enables the 3D printing of multilayered biopolymer hydrogels that can be integrated to design cell loaded constructs that are distinctly anisotropic.
Materials and Methods: 3t3 fibroblasts were encapsulated in 1% w/w gellan gum and were printed into specific shapes and sizes by using suspended manufacture bioprinting. After 14 days of culture a live/dead assay using calcein AM and propidium iodide was conducted to assess suitability of scaffolds as cell culture substrates.
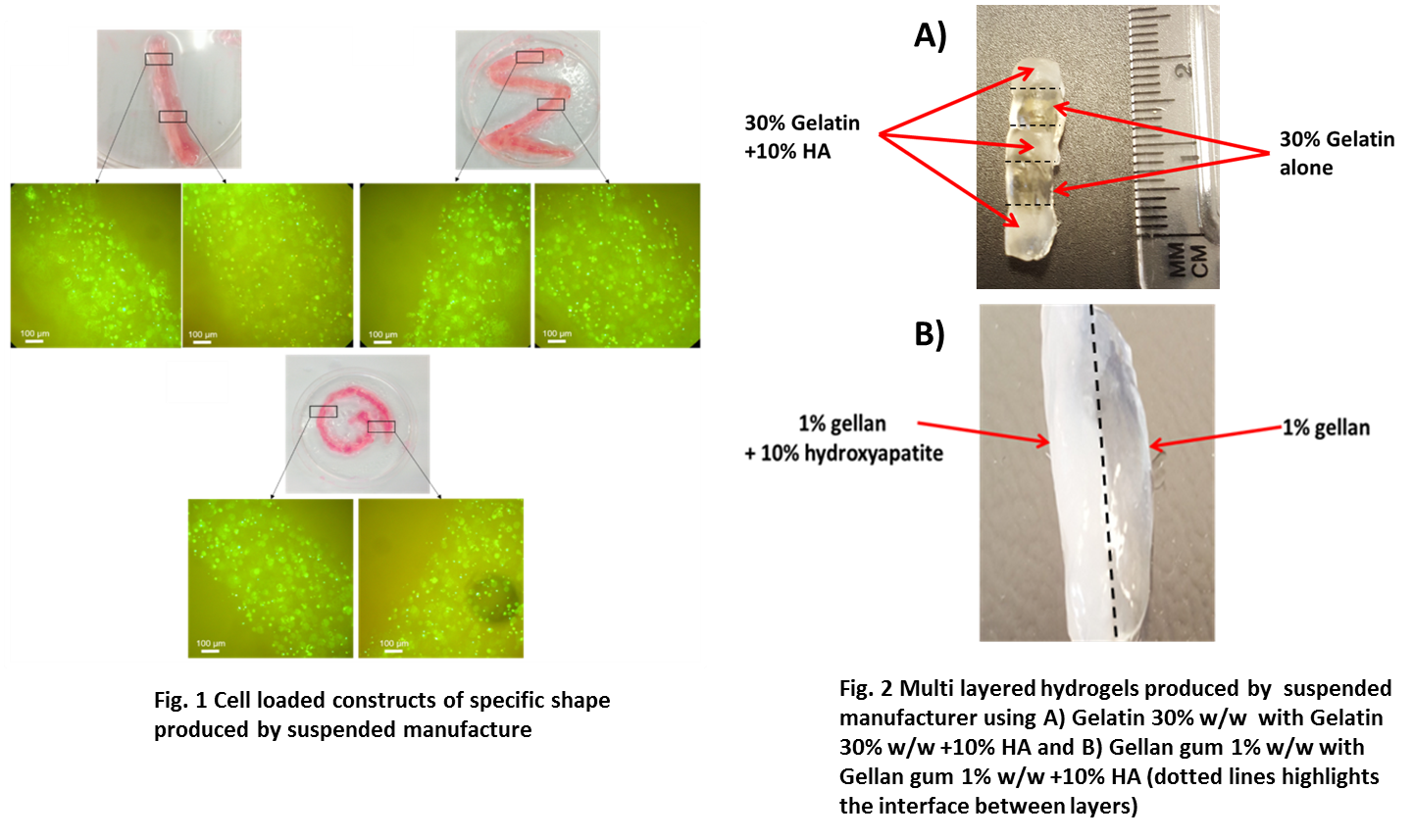
To print composite layered structures, 1% low acyl gellan was deposited upon 1% gellan containing 10% hydroxyapatite (HA) to model an osteochondral environment. Similar structures were also produced using 30% w/w gelatin containing 10% HA which was then alternately layered with 30% w/w gelatin without HA to create a structure with 5 distinct layers. Once the structures were printed gelation was initiated either by addition of 0.1% CaCl2 for the gellan gum structures or by thermally triggering gelation when using gelatin. Once gelled, the single composite constructs were removed and imaged.
Results and Discussion: Live/dead staining showed that cells can be cultured within bioprinted constructs produced using suspended manufacture (Fig. 1). Constructs of a specific shape were printed and live/dead images show clear boundaries of the bioprinted scaffolds and surrounding suspending media, highlighting that the cells remained alive, were immobilized within constructs and did not migrate from the gellan gum hydrogel. Fig. 2 illustrates that layered structures could also be produced out of different starting materials (gelatin and gelatin + HA (Fig 2A), gellan gum and gellan gum + HA (Fig 2B)). This is possible as the suspended manufacturing process allows the biopolymer solutions in the pre gel state to maintain the printed shape. Depositing materials upon one another in the solution state, enables the successful integration of materials that can be subsequently crosslinked, resulting in gelation of a single construct.
Conclusion: We have highlighted the potential of using a suspended manufacturing technique for 3D bioprinting cell loaded constructs of defined shape. Furthermore, biopolymer hydrogel constructs consisting of integrated layers were also produced using this technique to create an osteo-chondral ECM like structure. This method could facilitate the creation of intelligently designed cell-loaded structures containing a variety of materials, cell types and growth factors to more realistically mimic the in vivo environment. In addition the ability to control scaffold shape may have applications as implants to augment regeneration in multiple tissue defects.
The authors would like to thank the University of Huddersfield for funding the studentship for Sam Moxon