Introduction: One of the most predominant symptoms of skin aging is wrinkle formation. Current anti-aging and anti-wrinkle materials often induce a toxic response, which results in inflammation to increase tissue growth under the skin. Although effective, a much safer and more effective way to alleviate the effects of aging and thereby winkle formation needs to be developed. Several biomimetic peptide sequences[1] have been identified as structural mimics of collagen type I to be effective in stimulating the synthesis of key constituents of the extracellular matrix by fibroblasts, however, they typically suffer from poor skin penetration due to the existence of the stratum corneum. Incorporation of cell penetrating peptides has been an emerging strategy to transport a variety of cargo across cellular membranes in a dose dependent manner[2]. In this study, peptide amphiphiles that consist of both CPPs and biomimetic sequences were designed, synthesized and characterized. The abilities of these peptide amphiphiles to penetrate the skin, to reduce inflammation, and to promote fibroblast functions were determined.
Materials and Methods: Material characterization: Self-assembled peptide amphiphilic nanoparticles (APNP) were characterized by zeta potential to determine their charge, and by TEM to observe their size and morphology. Cell viability assay: To determine cell viability, human dermal fibroblasts (ATCC® PCS-200-011™) were seeded at a density of 10,000 cells/cm2 and were subsequently co-cultured with peptide amphiphiles at various concentrations for 24 hours. MTS assays were used to determine cell density after incubation. Cell adhesion and proliferation: To determine cellular adhesion/proliferation, human dermal fibroblasts (Lonza, CC-2511) were seeded at a density of 10,000 cells/cm2 and cultured in DMEM supplemented with 10% FBS and 1% P/S. Cells were incubated under standard cell culture conditions for a certain period of time (4h for adhesion; 1, 3, and 5 days for proliferation). The MTS assays were used to determine cell density after incubation. Bacteria growth curves: Bacteria were seeded in a 96-well plate at a density of 106 CFU/ml, and were then combined with varying APNP concentrations to obtain a final concentration of 0, 2, 4, 8, 12, 20, 40 and 80 µM. The well plate was then allowed to incubate at 37°C inside a spectrophotometer under static conditions. OD600 measurements were taken every 2 minutes for 24 hours to establish the speed of proliferation and shape of the bacterial growth curve. Skin penetration/permeation: Skin penetration and permeation were determined using the Static Franz Diffusion Cell (FD-C) with porcine skin as the membrane between donor and acceptor compartment due to their resemblance to human skin. Peptide amphiphiles were applied in the donor compartment, and PBS was used as the receptor solution. The skin samples were fixed and sectioned to examine penetration using confocal microscopy. All experiments were conducted in triplicate and repeated at least three different times. Statistical differences between means were determined using ANOVA followed by a student t-test where p < 0.01 was considered statistically significant.
Results and Discussion: The LC50 value of APNPs for human dermal fibroblasts was found to be around 76 µM, and no toxicity was identified for the same cells at a concentration of up to 12 µM (Figure 1). Also, it was found that these peptide amphiphiles as low as 2 µM in concentration have the ability to both delay the time it takes for bacteria to reach exponential growth phase and decrease bacteria density after one-day of culturing for gram positive bacteria (Figure 2). The effects increased in a dose dependent manner. Last, but not least, it was found out that APNPs were able to promote fibroblast cell proliferation as well as total collagen synthesis over the long term.
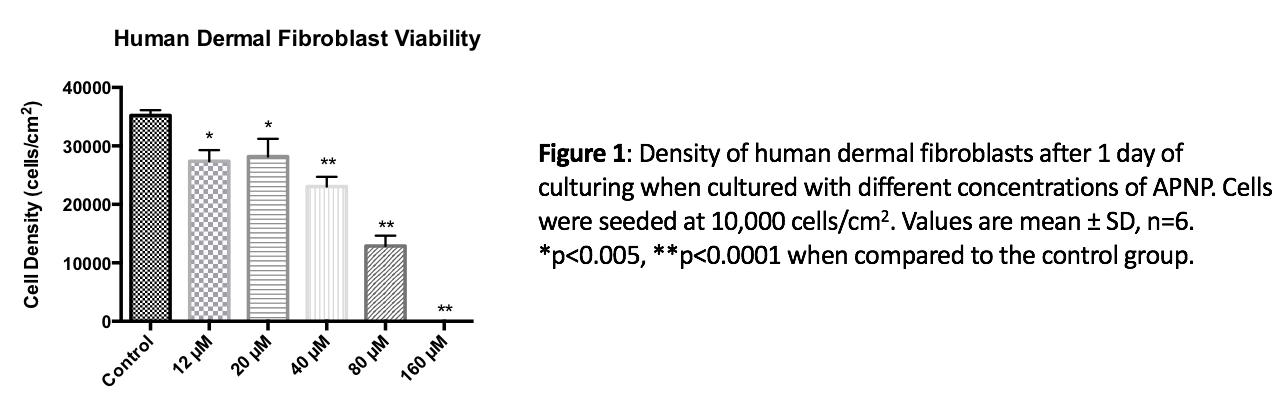
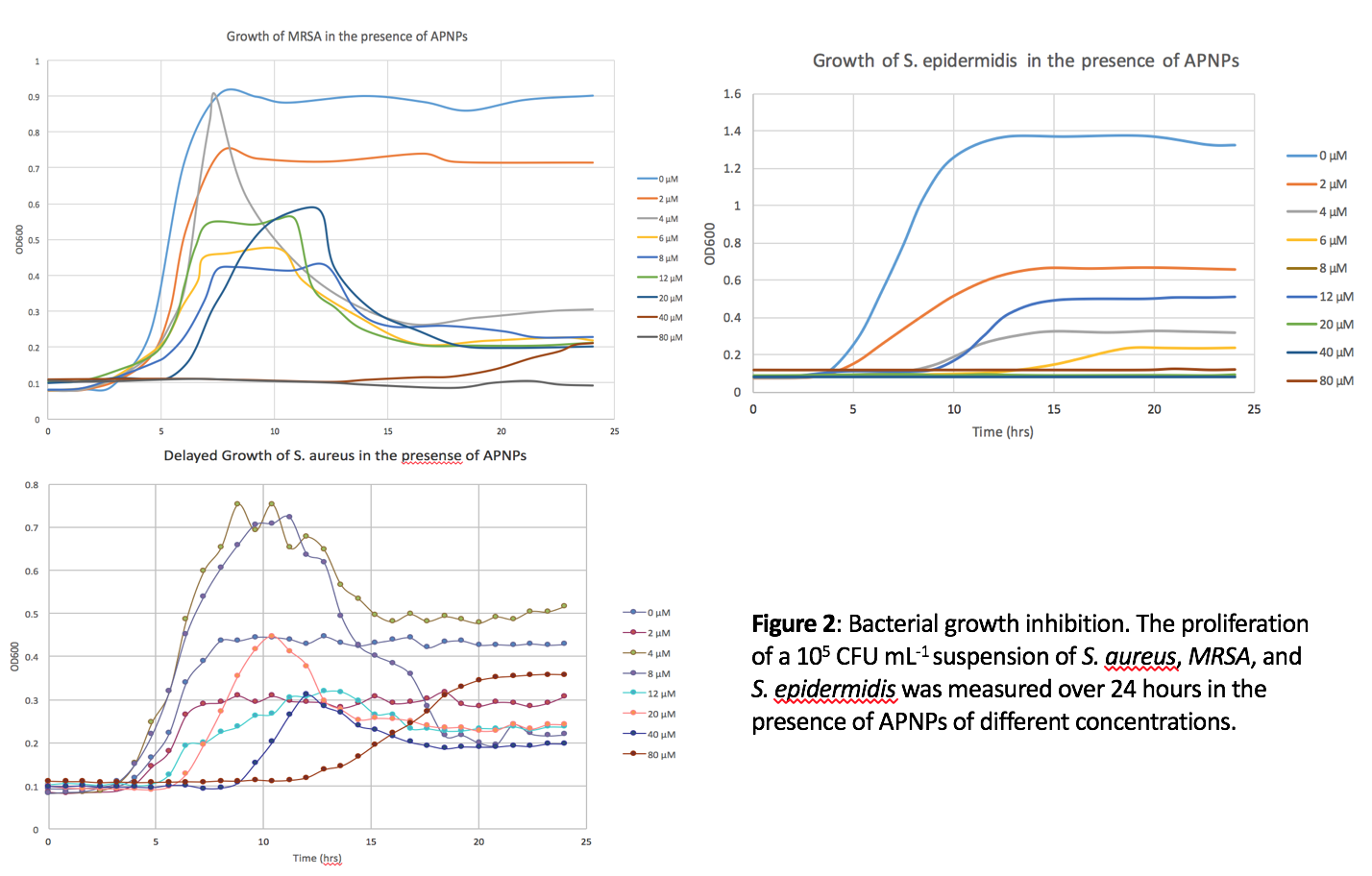
Conclusions: Through the above experiments, various peptide amphiphiles were identified that can be added to current skin formulations to promote the penetration of active ingredients, to increase fibroblast growth and to decrease bacteria growth, criteria necessary for improved anti-aging and antimicrobial agents.
The authors would like to thank Northeastern University for funding this research.
References:
[1] Zhao, Xiubo. et al. Molecular self-assembly and applications of desiner peptide amphiphiles. Chem. Soc. Rev., 2010, 39, 3480-3498.
[2] Heitz, F. el al. Twenty Years of Cell- Penetrating Peptides: From Molecular Mechanisms to Therapeutics. Br. J. Pharmacol. 2009, 157, 195–206.