Introduction: In situ crosslinkable hydrogels have received tremendous attention as a promising platform for tissue engineering and drug delivery. They can be injected into the target site via minimal invasive procedure and fill any irregular shapes of defects and tissues. In general, these hydrogels can be generated by various physical and chemical crosslinking reactions, including ionic interactions, hydrophobic interactions, Michael-type addition, photo-crosslinking and enzymatic reactions[1]. Among them, photo-initiation method is most frequently employed for the generation of hydrogels, however, this crosslinking system has still some limitations such as reagent toxicity, curing depth, and control of elastic modulus.
In this study, the gelatin hydrogels were prepared via fenton reaction using FeCl2, H2O2 and ascorbic acid. Fe2+ is approved from FDA, and H2O2 are safer and easily available than photo-initiation[2]. It was demonstrated that the gelation time and elastic modulus of hydrogels could be easily adjusted by FeCl2 and H2O2 concentrations. This hydrogel using fenton reaction is biocompatible enough to use biomedical applications.
Materials and Methods:
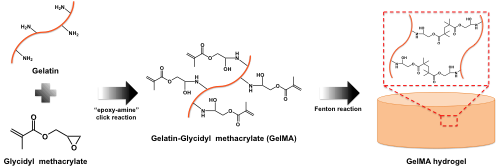
Fig 1. Synthetic route and crosslinking of GelMA.
Methacrylate grafted gelatin (GelMA) conjugates were synthesized by “epoxy-amine” click reaction between amine groups on gelatin and epoxy moiety on glycidyl methacrylate. The chemical structure of the GelMA was characterized by 1H NMR. The GelMA hydrogels were prepared in the presence of FeCl2, H2O2 and ascorbic acid. Thereafter, the physico-chemical properties (e.g., gelation time, mechanical strength and degradability) of hydrogels were characterized using a vial tilting method, rheological analysis and enzymatic degradation assay. In addition, in vitro cyto-compatibilty studies were carried out to evaluate cell viability using extracted solutions from the GelMA hydrogels.
Results and Discussion:
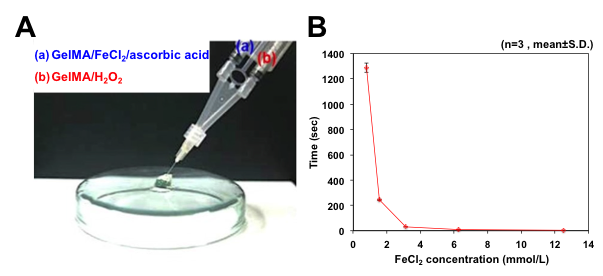
Fig 2. The picture of hydrogelation using fenton reaction (A) and the gelation time of GelMA hydrogels at various FeCl2 concentration (0.08~12.5 mmol/L) (B).
The chemical structure of GelMA was confirmed by 1H NMR, demonstrating glycidyl methacrylate was sucessfully conjugated into gelatin. We evaluated the physico-chemical properties of the GelMA hydrogels, such as gelation time and mechanical strength. The gelation time (5 sec-22 mins) and the mechanical strength (1-8.4 kPa) were easily tuned, depending on the concentrations of FeCl2 and H2O2. When collagenase (2 U/mL) was treated, increaing H2O2 resulted in a faster degradation rate. It may be probably due to the higher crosslinking density. Furthemore, in vitro cell study was carried out to investigate the cyto-compatiblity of the GelMA hydrogels formed by fenton reaction (FeCl2 concentration; 3.13-12.5 mM). exhibiting excellent cyto-compatibility (cell viability; 88-94 %, TCPS; 100%).
Conclusions
We have developed in situ crosslinkable gelatin hydrogels. produced by crosslinking using fenton reaction. It was demonstrated that crosslinking density and rate of hydrogels were controlable by varying FeCl2 and H2O2 concentrations. The prepared GelMA hydrogels were shown to have exellent degradability and cyto-compatibility. We expect that the fenton reaction have potential as a crosslinking system to fabricate injectable biocompatible hydrogels for biomedical applications.
This work was supported by Basic Science Research Program through the National Research Foundation of Korea(NRF) grant funded by the Ministry of Science, ICT & Future Planning (NRF-2015M3A9E2028577, 2015R1A2A1A14027221)
References:
[1] K.M. Park, K.S. Ko, Y.K. Joung, H. Shin, K.D. Park, J.Mater.Chem., 2011, 21, 13180.
[2] L. Sun, S. Zhang, J. Zhang, N. Wang, W. Liu, W. Wang, J.Mater.Chem. B, 2013, 1, 3932.