Introduction: Synthetic polymeric scaffolds mimic the structure of native extracellular matrix (ECM) but lack the biological activity of ECM. Cells cultured in synthetic scaffolds produce and assemble ECM, which harbors biological signals and can be incorporated into scaffolds to improve the biological activity of scaffolds. However, whether and how the composition of ECM derived from cell-culture regulates the biological function of scaffolds has been barely explored. In order to study the effect of the ECM composition on the biological activity of scaffolds, three different types of ECMs were prepared from freshly isolated fibroblasts, chondrocytes and osteoblasts cultured on electrospun fibrillar matrix scaffolds (fiber mats) and their effects on differentiation of human mesenchymal stem cells (hMSC) on scaffolds were analyzed. Also, the effect of such ECMs on porogen-leached 3D scaffolds (porous 3D scaffolds) was also evaluated and compared.
Methods: To modulate the ECM composition in scaffolds, fresh fibroblasts, chondrocytes and osteoblasts were isolated from bovine tissue. Primary cells were cultured on fiber mats or porous 3D scaffolds. Then ECMs on the scaffolds were prepared by an optimized decellularization method. The compositions of ECMs derived from different cell types were characterized using SDS-polyacrylamide gel electrophoresis, silver staining, immunostaining and glycosaminoglycans (GAG) assays. The chondrogenesis and osteogenesis of hMSC on various scaffolds were analyzed using qPCR and alkaline phosphatase (ALP) activity assay.
Results: Using an optimized decellularization method scaffolds with decellularized ECM were prepared from confluent cell cultures in scaffolds (Figure 1).
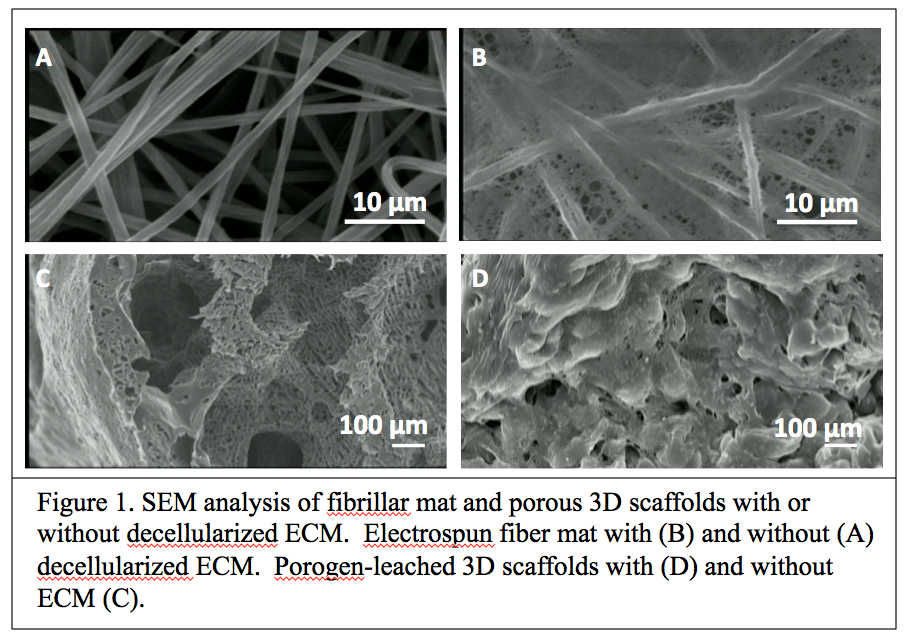
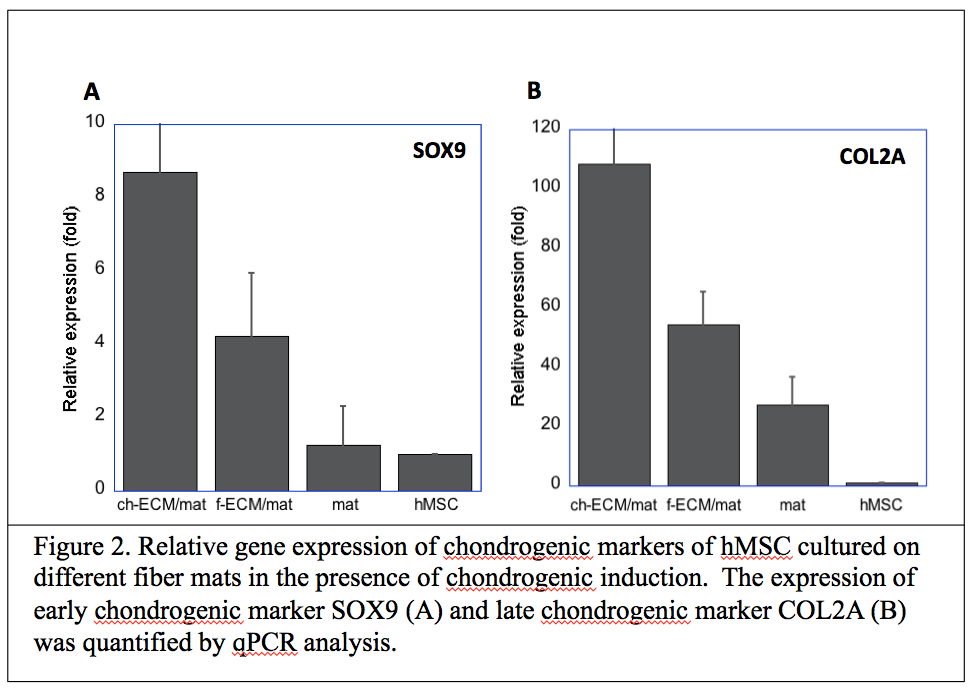
While the fiber mats containing chondrocyte-derived ECM (ch-ECM/mat) and fibroblast-derived ECM (f-ECM/mat) shared many common ECM components, they were also enriched with distinct ECM components. The GAG contents were higher in ch-ECM/mat than in f-ECM/mat. Type II collagen was present in ch-ECM/mats and was absent in f-ECM/mats. The chondrogenic and osteogenic differentiation of hMSC on various ECM/mats was evaluated. The ch-ECM/mat promoted better chondrogenic differentiation of hMSC compared with f-ECM/mat or plain mat in the presence (Figure 2) or absence of soluble growth factors.
In addition, hMSC cultured in ch-ECM/3D scaffolds also showed better chondrogenic differentiation compared with plain polymeric scaffolds or scaffolds with f-ECM. These observations consistently demonstrated the chondro-inductivity of scaffolds decorated with ECMs derived from chondrocytes.
ECM derived from the co-culture of osteoblast and chondrocytes on fiber mats promoted the osteogenic differentiation of hMSC compared with fiber mats containing chondrocyte ECM.
Discussion: This study demonstrated that decellularized ECMs derived from different cell types harbor distinct biological signals which guide distinct interaction between residing cells and the scaffolds. By incorporating ECM in polymeric scaffolds, it will be possible to design specific functional applications of polymeric scaffolds.