Introduction: Cell transplantation has been expected as a new medical treatment for diseases. However, simple cell injection into the damaged site is inefficient probably because the cell adhesion is not so high. The use of cell scaffold materials that supports cell adhesion, proliferation and differentiation is considered to be useful to resolve the problem. Keratin is the major structural fibrous protein of mammalian hair[1] and is not necessary to be considered the risk of biological contamination like BSE. Furthermore, RGD and LDV contained in keratin may support cell adhesion and growth[2]. In this study, keratin scaffolds derived from wool and human hair were prepared and evaluated for cell culture substrate and tissue engineering scaffold.
Materials and Methods: The water-soluble fraction of oxidized derivative of keratin was extracted from wool fibers and human hair by oxidative extraction. After oxidation by peracetic acid, they were put in Tris base solution to extract keratin. The extracted keratin solution was stirred vigorously, filtered to remove insoluble contents, and then precipitated by pH adjustment. The precipitates were freeze-dried and powderized. They were then redissolved in Tris base solution and processed into films and sponges by cast method and freeze-drying, respectively. These scaffolds were treated by glutaraldehyde to make water-insoluble. To investigate the biocompatibility of keratin in vitro, L929 cells were cultured on keratin films and sponges. For in vivo study, keratin films and sponges were subcutaneously implanted into Wistar rats.
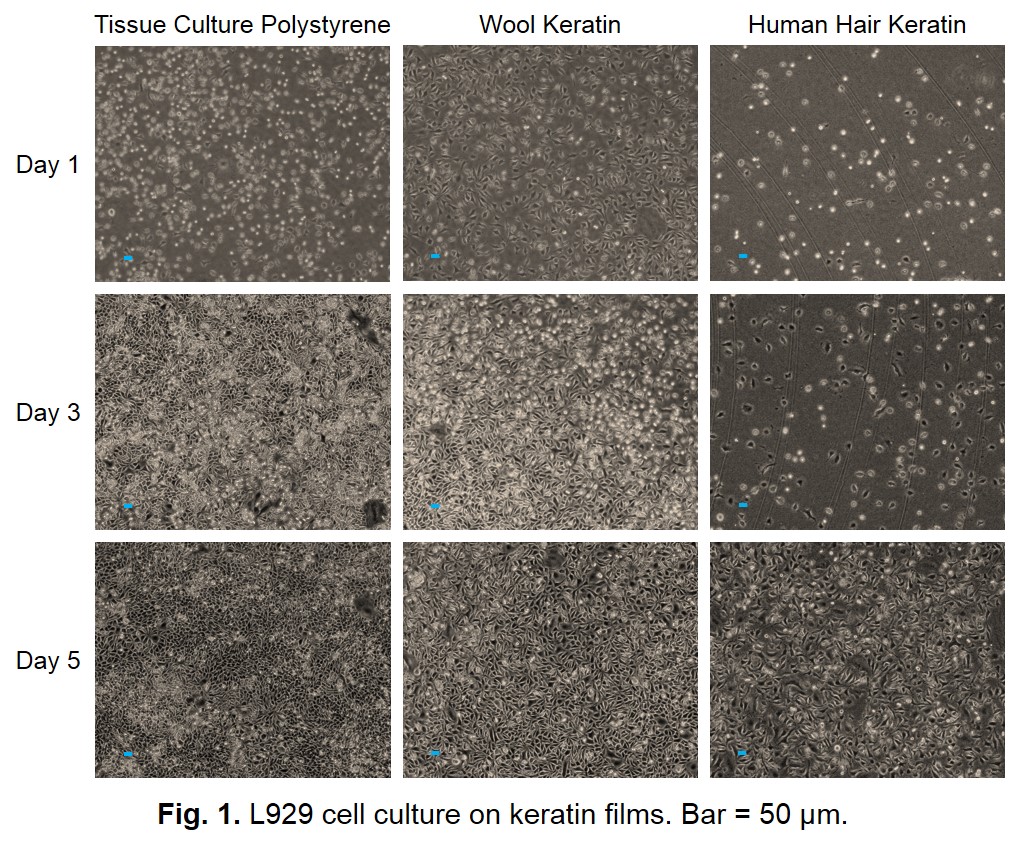
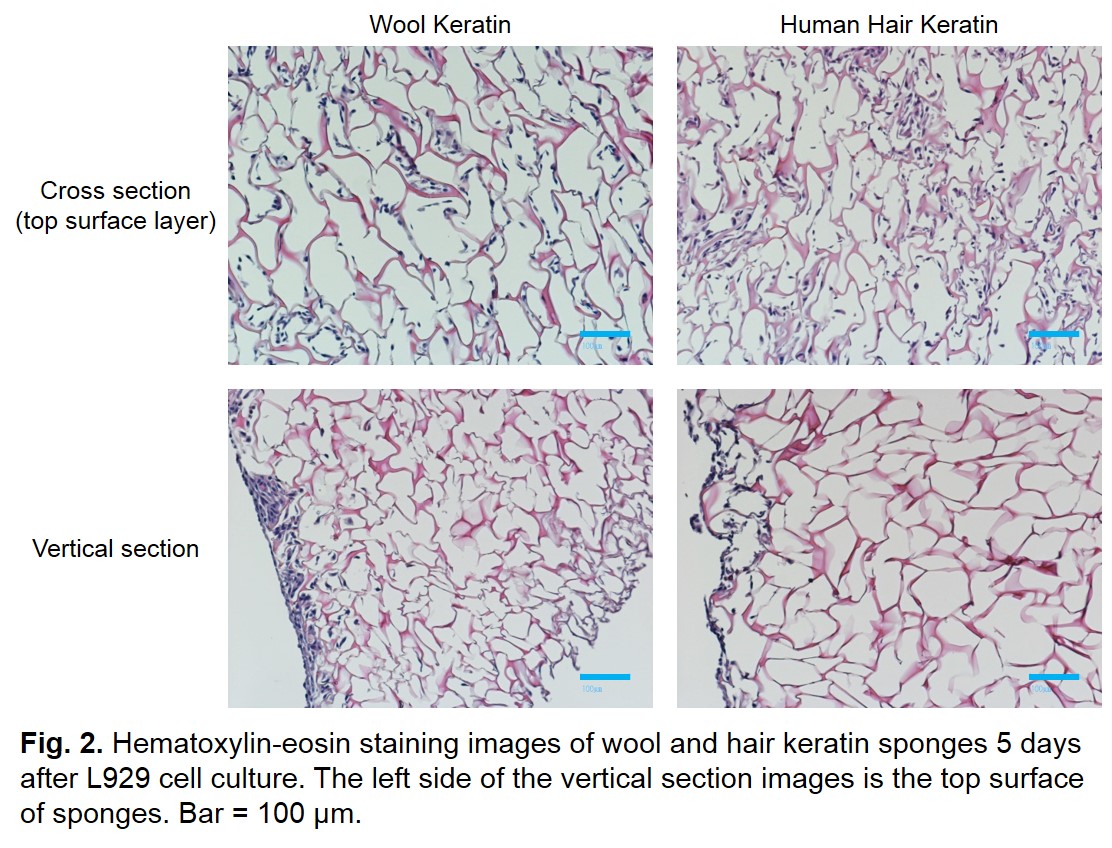
Results and Discussion: Wool and hair keratin films had moderate hydrophilic surface suitable for cell adhesion. Figure 1 shows the results of cell culture on the wool and hair keratin films. The L929 cells were grown well and their growth rate was almost equivalent to that on the tissue culture dish. Figure 2 shows the hematoxylin-eosin staining images of the wool and hair keratin sponges after in vitro cell culture. Although the L929 cells were grown on surface layer of sponges, cells were not observed inside of them. This result suggests that the sponge needs to be given a communicating internal structure with each other to make it possible for cells to infiltrate into inside of the scaffold more deeply. The wool keratin films and sponges implanted subcutaneously into rats were extracted at 1, 2, 4 weeks. The films were not observed significant change in size. Moreover, infiltration of the recipient cells and vascularization was observed.
Conclusion: The results of cell culture on the wool and hair keratin scaffolds showed that keratin was a suitable substrate for cell culture. In general, keratin can be obtained from non-vascular tissue which is irrelevant to the problem of BSE. In addition to good biocompatibility, keratin source has the advantages of readily available and less expensive. Further detail investigations would raise the potential of keratin as a useful biomaterial.
References:
[1] Feughelman M. Keratin. Encyclopedia of Polymer Science and Engineering, 2nd Edition, Wiley. 2nd ed. New York: Wiley; 566, 1985.
[2] Tachibana A, Furuta Y, Takeshima H, et al. Fabrication of wool keratin sponge scaffolds for long-term cell cultivation. J Biotechnol, 93(2), 165, 2002.