Introduction: Polyethylenimines (PEIs) are considered the gold standard polymeric gene delivery vectors[1]. However, conflicting results on their performance are reported owing to the wide variability of experimental setups employed across laboratories[2]. With this in mind, herein we investigated the influence of the complexation protocol and of the transfection medium on the properties and on the activity of PEI-based polyplexes.
Materials and Methods: Polyplexes were prepared by combining plasmid DNA (pGL3 or pEGFP) and linear or branched PEI (lPEI, bPEI, respectively, MW 25 kDa) adding reagents dropwise (DROPPING) or by mixing by pipetting (MIXING). The final N/P ratio was 30 and both 10 mM Hepes pH 7 and 150 mM NaCl were used as complexation medium. Hydrodynamic diameters (DH) were measured by DLS before and after dilution in cell culture medium containing or not 10% FBS. For transfections, 2 x 104 HeLa cells/cm2 were seeded and 24 h later, polyplexes were added. In some experiments cells were centrifuged at 1,000 g after polyplex addition or transfected upside-down (seeded surface suspended and facing the bottom of the well). 24 h after transfection, cytotoxicity was evaluated by AlamarBlue and transfection efficiency by Luciferase Assay System as previously described[3].
Results and Discussion: The method of addition of reagents strongly affected the dimensions of polyplexes for all the conditions tested, except for lPEI in NaCl. Particularly, by adopting the MIXING method, polyplexes showed DH < 200 nm with narrow size distributions (PDI < 0.2). Following the DROPPING protocol, DH and PDI increased significantly, indicating the formation of bigger aggregates. Inversely, lPEI in NaCl always gave rise to big, polydisperse particles (DH > 1 µm, PDI ≈ 1). Upon addition of culture medium a common trend was observed: polyplex dimensions were roughly stable in the presence of 10% FBS while without serum they increased up to values > 1 µm within the first 2 h.
Except for lPEI in NaCl, polyplex preparation by the DROPPING protocol induced a strong increase of transfection efficiency as compared to MIXING (≥ 2 times) when transfections were carried out in complete medium (Fig. 1).
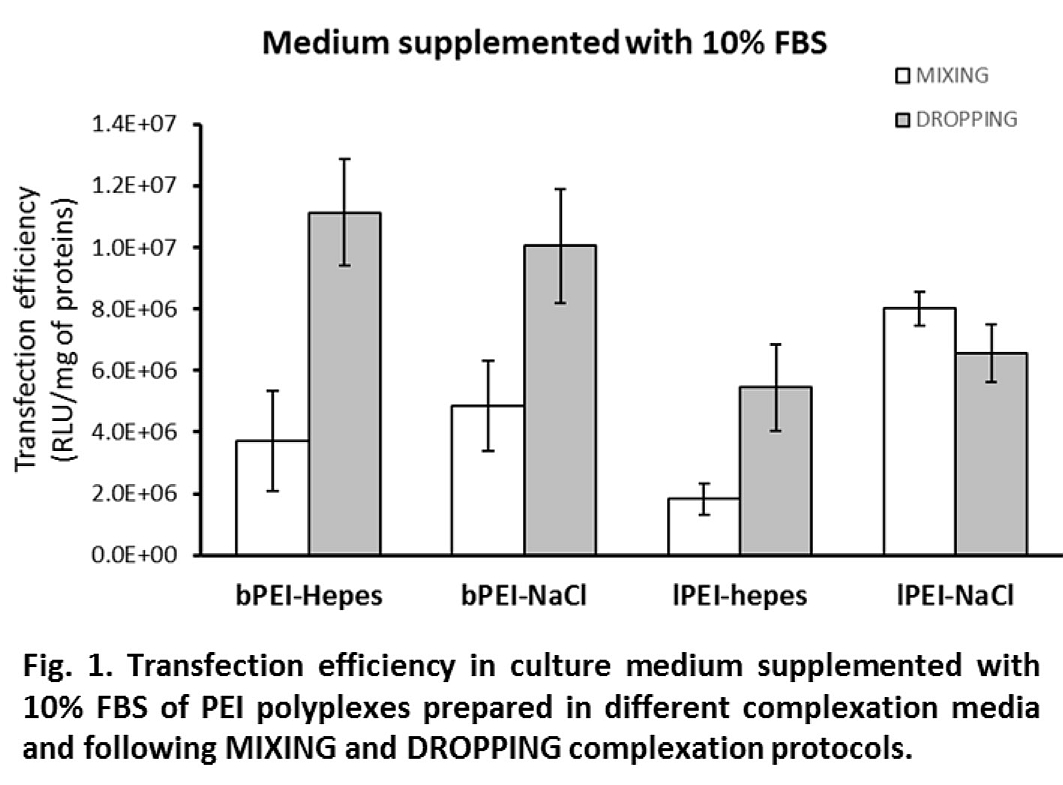
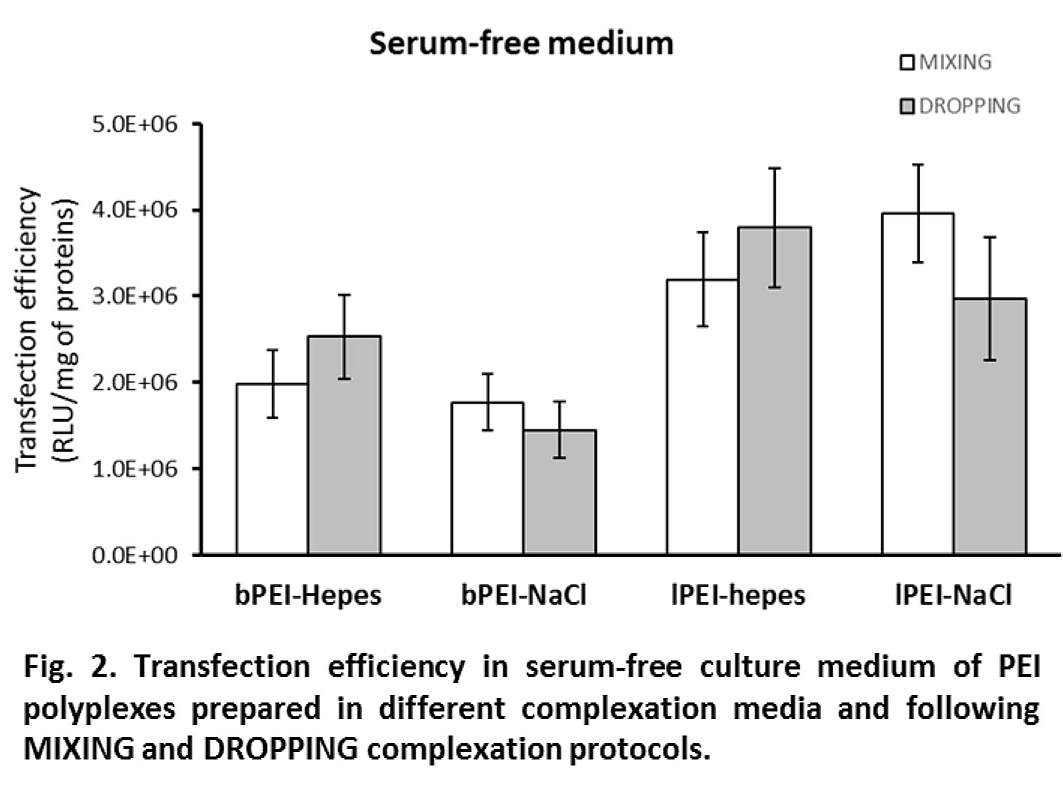
Interestingly, differences in efficiency dropped or even disappeared when experiments were performed in the absence of FBS (i.e., in OptiMEM, Fig. 2). In 10% FBS, where the DH was stable, a direct relationship between DH and transfection activity can be noticed: bigger polyplexes showed higher transfection levels; in serum-free medium, the growth of DH led to big polyplexes which showed comparable gene delivery activity. Cytotoxicity was always lower than 20% except for bPEI polyplexes administered in serum-free medium for which it increased to values between 30 and 40%.
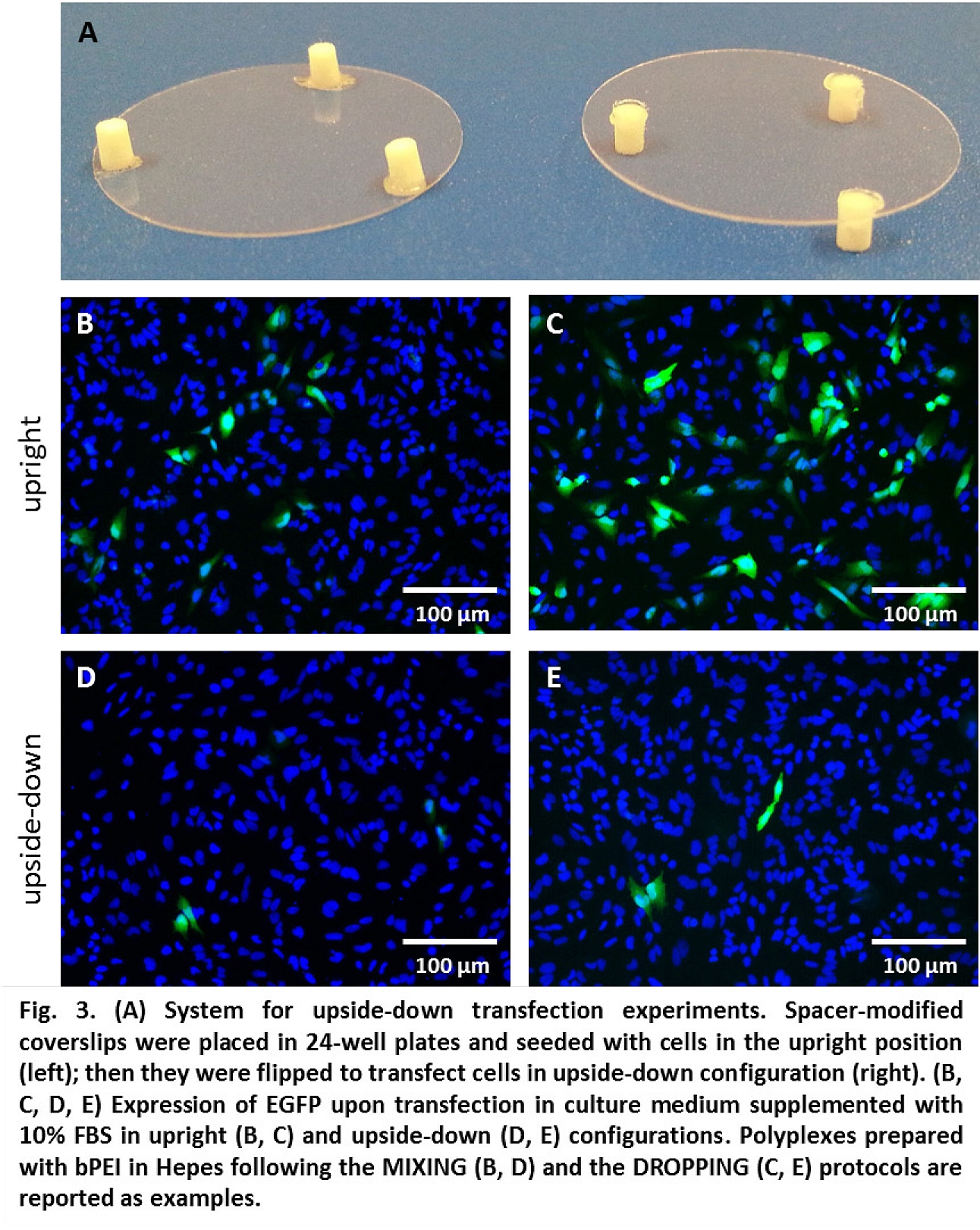
Centrifugation of polyplexes over cells (to increase sedimentation) boosted transfection efficiency decreasing the difference in activity between DROPPING and MIXING protocols. Oppositely, in experiments with cells cultured upside-down (no sedimentation over cells), transfection strongly diminished (Fig. 3), pointing out the importance of gravitational sedimentation of complexes.
Conclusion: Herein we described how the complexation protocol may influence the properties of PEI polyplexes, highlighting a relationship between DH and transfection efficiency and identifying sedimentation over cells as a key driving force in their transfection process.
This work was partially supported by the Italian Ministry of Education, University and Research (MIUR-FIRB, Grant Number: RBFR08XH0H) and by NSERC-Canada; DP was awarded of a postdoctoral scholarship by NSERC CREATE program for regenerative medicine (NCPRM, www.ncprm.ulaval.ca)
References:
[1] Pezzoli D, Candiani G (2013). J Nanopart Res 15 (3):1523.
[2] Malloggi C, Pezzoli D, Magagnin L, De Nardo L, Mantovani D, Tallarita E, Candiani G (2015). Polymer Chemistry 6:6325-6339.
[3] Pezzoli D, Kajaste-Rudnitski A, Chiesa R, Candiani G (2013). Methods Mol Biol 1025:269-279.