Introduction: Over 500,000 people are diagnosed with head and neck cancers per year worldwide. Radiation therapy for these cancers causes extensive and permanent damage to secretory acinar cells within the salivary glands leading to permanent dry mouth for which no curative therapy exists[1]. Cell-based therapies developed by the suspension culture of primary submandibular gland (SMG) cells has shown efficacy in restoring function after gland irradiation. However, regeneration is variable and the mechanism resulting in partial acinar regeneration in vivo is unclear[2],[3]. To control acinar cell survival and function for subsequent transplantation in vivo, we are developing biomimetic poly(ethylene glycol) (PEG) hydrogels to culture primary SMG cells.
Materials and Methods: SMG cells were isolated from mouse submandibular glands as described previously[4]. SMG cells form multicellular aggregates termed “spheres” after 48- hour suspension culture. SMG spheres were encapsulated in thiol-ene polymerized hydrogels with either matrix metalloproteinase (MMP) or hydrolytic degradability and cultured for 14 days in vitro. Expression of acinar (Mist1 and Aqp5) and duct (Cp2L1) cell markers was assessed by qPCR. Cell morphology was assessed by F-actin and nuclear staining. Production of a marker of salivary gland function, α-amylase, was measured by ELISA.
Results and Discussion: Imaging reveals that initially the majority of SMG cells have a rounded or columnar appearance (Fig 1A), consistent with the morphology of acinar cells. However, after sphere formation, primary SMG cells have larger nuclei and a cuboidal appearance (Fig 1B), similar to duct cells. Lineage tracing experiments have demonstrated that during sphere formation ~80% of the cell population consists of acinar cells[4]. However, primary SMG cells experience a 50-100 fold drop in expression of key acinar cell markers during the 48 hours of sphere formation (Fig 1C), suggesting that acinar cells are undergoing de-differentiation. In vitro culture of pancreatic acinar cells shows trans-differentiation to duct cells[5]. Similarly, SMG spheres encapsulated within hydrolytically degradable PEG hydrogels exhibited a ~20-fold increase in a duct marker, while acinar markers exhibit no increase (Fig 1D), suggesting an increase in duct cell phenotypes.

As acinar cells are the cells destroyed by radiation[6], we sought to incorporate MMP-degradability within the hydrogels as a means to mimic the native ECM and promote acinar cell differentiation. SMG spheres encapsulated within MMP-degradable PEG hydrogels demonstrate an increase in acinar markers (Figs 2A-B). Additionally, SMG spheres encapsulated within MMP-degradable hydrogels form acinar-like structures (Fig 2C) after 7 days. Future experiments will employ genetic lineage tracing of acinar cells to demonstrate acinar to duct trans-differentiation. Acinar cell phenotype will be confirmed with immunofluorescence and analysis of other secreted factors.
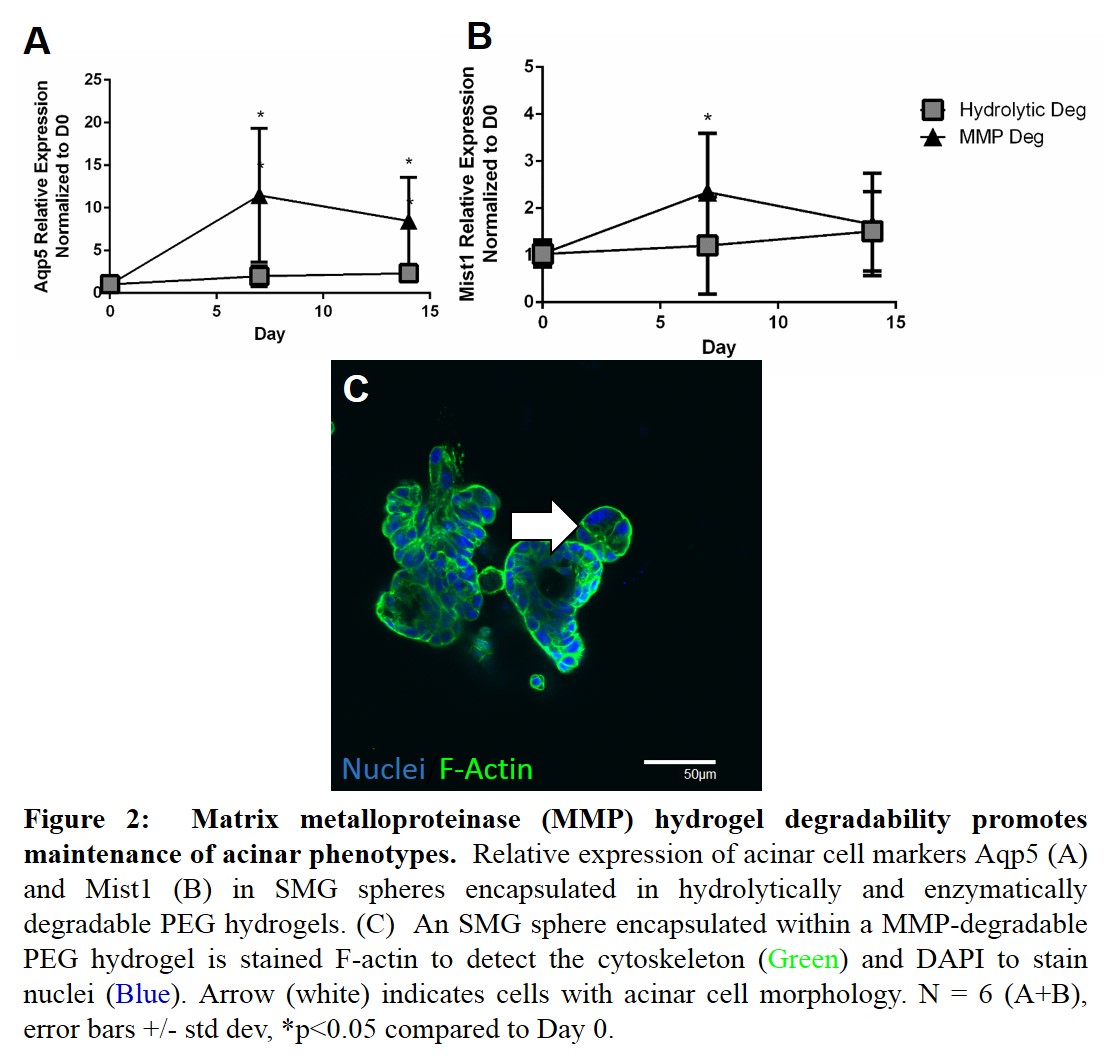
Conclusion: Successful salivary gland tissue engineering strategies require functional acinar cells. However, primary culture of submandibular gland cells results in a large decrease in acinar cells, as highlighted by downregulated acinar markers and changes in cell morphology. SMG spheres encapsulated within hydrolytically-degradable PEG hydrogels have an increase in a duct cell markers, suggesting acinar to duct cell trans-differentiation. Incorporation of MMP-degradability promotes the expression of acinar gene markers, underlying the importance of a mimetic extracellular niche to provide cues required for regenerating salivary gland cell types.
The authors would like to thank Linda Callahan and the University of Rochester Confocal Microscopy Core for there assistance in imaging. Funding provided by: R01 DE022949 and F30 CA183320.
References:
[1] Jensen, S.B., et al., A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer, 2010. 18(8): p. 1061-79.
[2] Lombaert, I.M., et al., Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One, 2008. 3(4): p. e2063.
[3] Aure, M.H., S. Arany, and C.E. Ovitt, Salivary Glands: Stem Cells, Self-duplication, or Both? J Dent Res, 2015.
[4] Shubin, A.D., et al., Development of poly(ethylene glycol) hydrogels for salivary gland tissue engineering applications. Tissue Eng Part A, 2015.
[5] Means, A.L., et al., Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development, 2005. 132(16): p. 3767-76.
[6] Guchelaar, H.J., et al. Radiation-induced xerostomia: pathophysiology, clinical course and supportive treatment. Support Care Cancer, 1997. 5(4): p. 281-8.