Introduction: An emerging strategy in bone tissue engineering is to fabricate scaffolds of structurally simpler resorbable materials which support and encourage inherent or implanted cells to remodel the scaffold into more complex natural bone.[1] Alginate is a biopolymer derived from seaweed which forms hydrogels in the presence of divalent cations which makes it suitable for cell encapsulation.[2] Previously we have developed alginate-calcium phosphate (CaP) composites showing great potential as bone scaffolds.[3]
In this work the in situ formation and temporal changes in mineral of an alginate-CaP composite was studied with a range of physiochemical analysis techniques on a platform consisting of a simple flow cell. Mineralization of alginate with dicalcium phosphate dihydrate (DCPD) was achieved by the use of crystal seeds to control mineral formation and supersaturation.[4] Our newly developed experimental platform allowed for detailed study of both formation of DCPD and its conversion into hydroxyapatite (HAp).[5]
Experimental Methods: A 1.5 µL drop of alginate with or without DCPD-seeds and a phosphate precursor were placed between a microscope slide and cover slip separated by 200-300 µm. A CaCl2 gelling solution was then introduced into the cell to initiate gelling and mineral formation.
Mineral formation and local pH evolution were monitored using optical microscopy (Olympus IX70) and confocal microscopy (Leica SP5) of solutions containing pH-sensitive fluorescent dyes.
The formed mineral was studied by Raman microscopy (Renishaw InVia Reflex) to determine its phase and distribution within the hydrogel. A selection of samples were critical point dried (Emitech K850) and imaged using SEM (Hitachi S-5500 S(T)EM).
Seeded samples were incubated in SBF in order to assess the bioactivity of the composite.
Results and Discussion:
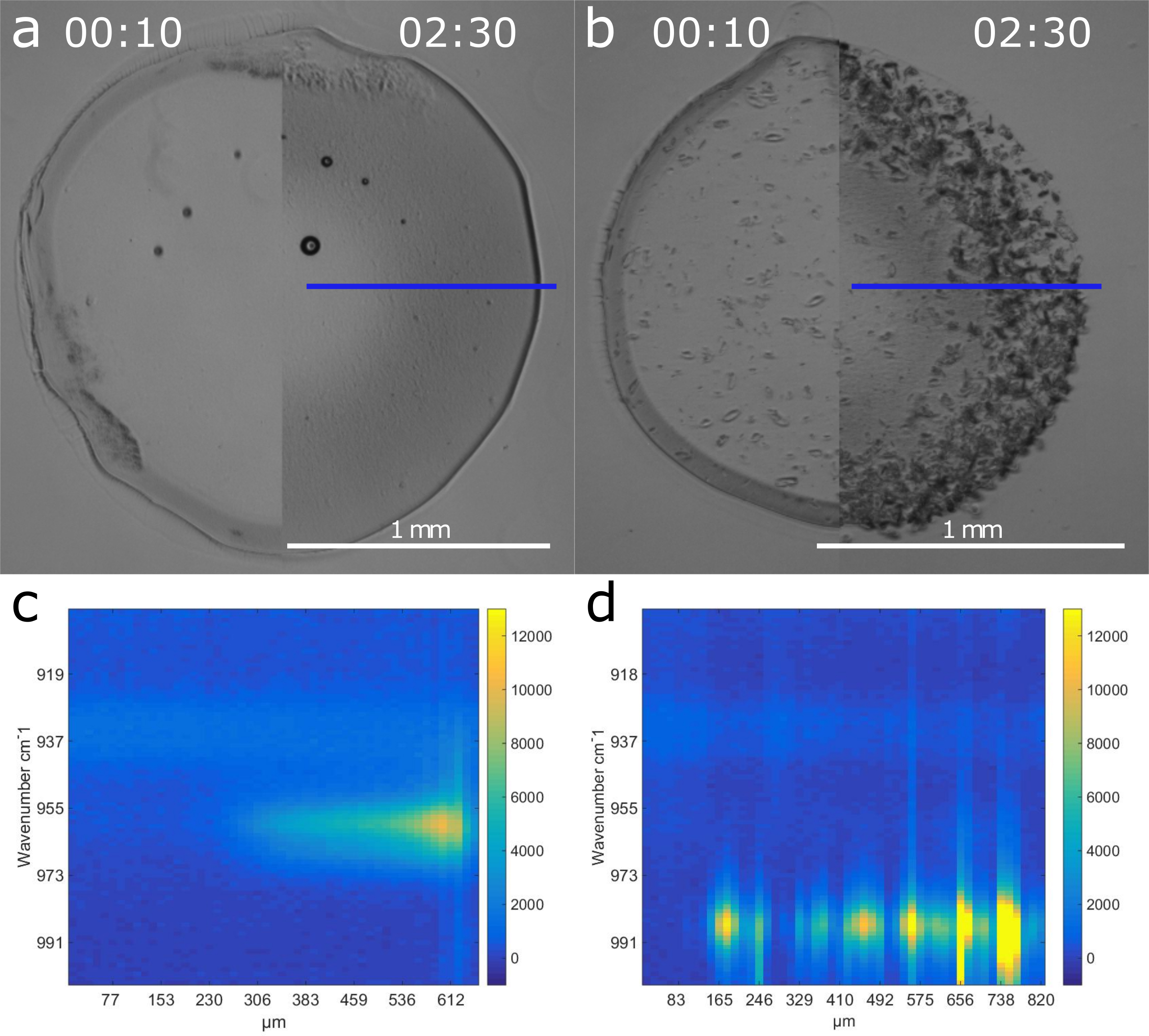
Figure 1: Micrographs showing the difference between unseeded (a) and seeded (b) mineralization with corresponding Raman line scans (approx. position given by the blue lines) including the main phosphate peak for HAp (c) and DCPD (d) after 1 h.
The incorporation of DCPD-seeds greatly influenced the mineralization process of the hydrogel, not only by providing nucleation sites, but also through secondary nucleation and a change in supersaturation conditions. As shown by Raman microscopy, at otherwise similar conditions, the resulting mineral phase changed from HAp or octacalcium phosphate (OCP) nanocrystals for unseeded samples to much larger DCPD platelets for the seeded samples. As can be seen in Figure 1, these crystals are far more numerous than the initial seeds, indicating the nucleation of new crystals.
DCPD is known to be more resorbable than HAp, due to its higher solubility and samples incubated in SBF showed conversion into HAp for the seeded samples. High-resolution SEM-imaging revealed a morphological change in the HAp-crystals from flaky platelets to needle-like bundles during maturation.
Conclusion: The results shed light onto processes involved in mineralization and DCPD transformation within hydrogel networks and may aid in the process of designing more bioactive scaffold materials.
We acknowledge the Research Council of Norway for financial support (FRINATEK project 214607).
References:
[1] Bose, S.; Roy, M.; Bandyopadhyay, A., Recent advances in bone tissue engineering scaffolds. Trends in biotechnology 2012, 30 (10), 546-554.
[2] Andersen, T.; Strand, B. L.; Formo, K.; Alsberg, E.; Christensen, B. E., Chapter 9 Alginates as biomaterials in tissue engineering. In Carbohydrate Chemistry: Volume 37, The Royal Society of Chemistry: 2012; Vol. 37, pp 227-258.
[3] Xie, M.; Olderoy, M. O.; Zhang, Z.; Andreassen, J.-P.; Strand, B. L.; Sikorski, P., Biocomposites prepared by alkaline phosphatase mediated mineralization of alginate microbeads. RSC Advances 2012, 2 (4), 1457-1465.
[4] Bjørnøy, S. H.; Bassett, D. C.; Ucar. S.; Andreassen, JP.; Sikorski, P., Controlled mineralisation and recrystallisation of dicalcium phosphate dihydrate within alginate hydrogels. Submitted to Biomedical materials
[5] Bjørnøy, S. H.; Bassett, D. C.; Mandaric, S.; Sikorski, P., In situ investigations of chemical composition, morphology, gelling and mineralization kinetics in hydrogel/mineral composites. 27th European conference on biomaterials, Krakow 2015.