Cardiovascular pathologies represent one of the main causes of death and morbidity in the world[1]. Fucoidans, sulfated polysaccharides extracted from brown algae are versatile biomolecules endowed with numerous biological activities[2]-[7]. In particular, a low molecular weight fucoidan (LMWF), able to strongly bind to P-selectin, allowed the diagnosis of vascular thrombosis and ischemia by SPECT imaging with 99mTc in rat models[8]-[10].
The radiolabeling studies proceeded by straightly mixing a saline solution of LMWF with 99mTcO4- in appropriate conditions without any chemical modification of the polysaccharide[9]. As part of a joint laboratory with the French company Algues & Mer which produces the LMWF, our works aim to obtain a contrast agent kit for the clinical SPECT imaging of atherothrombosis and heart ischemia.
Materials and Methods: LMWF is prepared by an aqueous treatment of Ascophyscient®, a polysaccharidic extract from Ascophyllum nodosum (Algues & Mer, Ouessant, France)[10]. Molecular weights were determined using HPSEC-MALLS-dRI[9]. Colorimetric assays led to fucose[11], uronic acid[12] and sulfate[13] contents and the neutral sugar composition was obtained by acid hydrolysis of LMWF and HPAEC analysis with amperometric detection. The contrast agent was prepared by mixing a solution of ascorbic acid, tin chloride and sodium chloride and another one containing the LMWF and sodium chloride. 99mTcO4- was added just before injection.
Results and Discussion: Fucoidans are natural polymers and their chemical compositions vary depending on the species, water temperature and the harvest period of the algae. For the preparation of a clinical contrast agent, LMWF had to present well-defined and reproducible physicochemical features. Numerous batches of LMWF have been purified from Ascophyscient® and analyzed to provide these parameters.
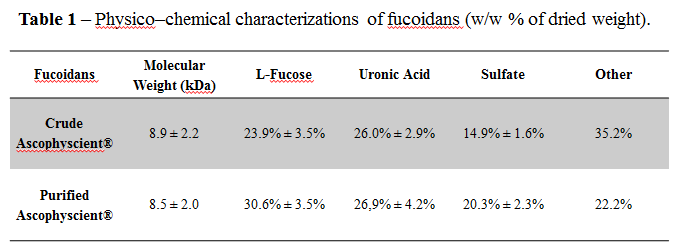
The average molecular weights were about 8,000 Da. Fucose and sulfate, as part of the key features responsible for the biological activities of fucoidans[14],[15], and uronic acids constitute about 80% (w/w) of the polymer, with a few amounts of xylose, glucose, galactose and mannose as the remaining 20%.
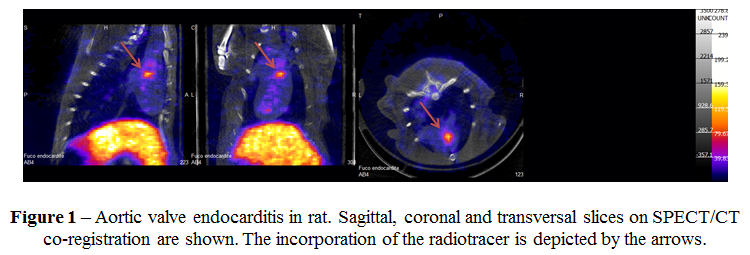
A radiolabelling kit was prepared, all reactants being kept lyophilized until the radiolabelling with a saline solution of 99mTcO4-. Purity was >95%. The biodistribution study in rat of both compounds labeled with 99mTc evidenced a predominant renal elimination of the purified fucoidan, whereas the crude fucoidan (Ascophyscient®) was mainly retained in liver and spleen. SPECT imaging of rat models have shown the effectiveness and the selectivity of 99mTc-LMWF toward pathological myocardium (figure). All this work is part of a large-scale european project (NanoAthero).
Conclusion: This purified sulfated polysaccharide appears promising for the development of molecular imaging in acute coronary syndrome and a clinical trial will start on 2016.
ANR LabCom; ANR FucoThrombo; ANRT for CIFRE fellowship n°2014/0337 (Lucas Chollet); Large-scale integrated project NanoAthero (NMP-2012-309820)
References:
[1] Finegold, J.A.; Asaria, P.; Francis, D.P. Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations. Int. J. Cardiol. 2013, 168, 934–945.
[2] Chaubet, F.; Chevolot, L.; Jozefonvicz, J.; Durand, P.; Boisson-Vidal, C. Retationships between chemical characteristics and anticoagulant activity of low molecular weight fucans from marine algae. B.S.P aulsen( ed.). Bioact. Carb. Polymers. 2000. 59-84.
[3] Maka, W.; Hamida, N.; Liua, T.; Lua, J.; Whitea, W.L. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydrate Polymers. 2013, 95, 606– 614.
[4] Abu, R.; Jiang, Z.; Ueno, M.; Isaka, S.; Nakazono, S.; Okimura, T.; Cho, K.;Yamaguchi, K.; Kim, D.; Oda, T. Anti-metastatic effects of the sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum on B16 melanoma. Biochem Biophys. Res. Comm.. 2015, 1-6.
[5] Mourão, P.A.S. Perspective on the Use of Sulfated Polysaccharides from Marine Organisms as a Source of New Antithrombotic Drugs. Mar. Drugs 2015, 13, 2770-2784.
[6] Atashrazm, F.; Lowenthal, R.; Woods, G.; Holloway, A.; Dickinson, J. Fucoidan and Cancer: A Multifunctional Molecule with Anti-Tumor Potential. Mar. Drugs. 2015, 13, 2327-2346.
[7] Han, Y.S.; Hee Lee, J.; Hun Lee, S. Antitumor Effects of Fucoidan on Human Colon Cancer Cells via Activation of Akt Signaling. Biomol. Ther. 2015, 23(3), 225-232.
[8] Bachelet, L.; Bertholon, I.; Lavigne, D.; Vassy, R.; Jandrot-Perrus, M.; Chaubet, F.; Letourneur, D. Affinity of low molecular weight fucoidan for P-selectin triggers its binding to activated human platelets. Biochim Biophys Acta 1790. 2009, 141–146.
[9] Rouzet, F.; Bachelet-Violette, L.; Alsac, J.M.; Suzuki, M.; Meulemans, A.; Louedec, L.; Petiet, A.; Jandrot-Perrus, M.; Chaubet, F.; Michel, J.B.; Le Guludec, D.; Letourneur, D. Radiolabeled Fucoidan as a P-Selectin Targeting Agent forIn Vivo Imaging of Platelet-Rich Thrombus and Endothelial Activation. J Nucl Med. 2011, 52 (9), 1433-1440.
[10] Saboural, P.; Chaubet, F.; Rouzet, F.; Al-Shoukr, F.; Azzouna, R. B.; Bouchemal, N.; Picton, L.; Louedec, L.; Maire, M.; Rolland, L.; Potier, G.; Le Guludec, D.; Letourneur, D.; Chauvierre, C. Purification of a Low Molecular Weight Fucoidan for SPECT Molecular Imaging of Myocardial Infarction. Mar. Drugs. 2014, 12, 4851-4867.
[11] Dische, Z. New Color Reactions for Determination of Sugars in Polysaccharides. In Methods of Biochemical Analysis; Glick, D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1955, 2, 313–358.
[12] Bitter, T.; Muir, H.M. A modified uronic acid carbazole reaction. Anal. Biochem. 1962, 4, 330–334.
[13] Gustafsson, L. Determination of ultramicro amounts of sulphate as methylene blue—II The reduction. Talanta 1960, 4, 236–243.
[14] Pomin, V.H. Fucanomics and galactanomics: Current status in drug discovery, mechanisms of action and role of the well-defined structures. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 1971–1979.
[15] Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology. 2003, 13, 29R–40R.