Introduction: Bacterial infection after implantation of a corneal implant is a serious complication and an economic burden. Conventional antibiotic prophylaxis, such as topical vancomycin application, is limited by low bioavailability, high dosing requirement and poor patient compliance[1],[2]. An ideal solution to overcome these issues is an antibiotic-eluting implant that sustains the local antibiotic delivery. In this study, we incorporated vancomycin in collagen hydrogel (VH) to create an artificial cornea with anti-infective capability.
Materials and methods: Vancomycin was loaded in 15%(w/v) bovine collagen type I collagen hydrogels with 0.2 mm thickness and 5 mm diameter. Release profile of the VH and minimum inhibitory concentration (MIC) of the drug released in the PBS medium against Staphylococcus aureus on day 1, 3, 5, 7 and 10 were first assessed. Clear medium in wells indicated complete inhibition of vancomycin against S. aureus at respective drug concentration. The VH was then implanted intrastromally following femtosecond laser-assisted small incision lenticule extraction in rabbits. In vivo biocompatibility of the implants was followed up to 1 month post-implantation using an in vivo confocal microscope (n=3). In a separate group of rabbits (n=3), 50 μl of 108 CFU/ml S. aureus inoculate was injected intrastromally on day 2 post-implantation and the performance of the implants was followed up for 3 further days. Blank hydrogel (BH) was implanted in contralateral eyes for comparison. Follow-up observations were performed by slit lamp photography and optical coherence tomography. After the rabbits were sacrificed and corneas were dissected, bacterial quantification and immunohistochemistry of inflammatory markers, CD18, were carried out.
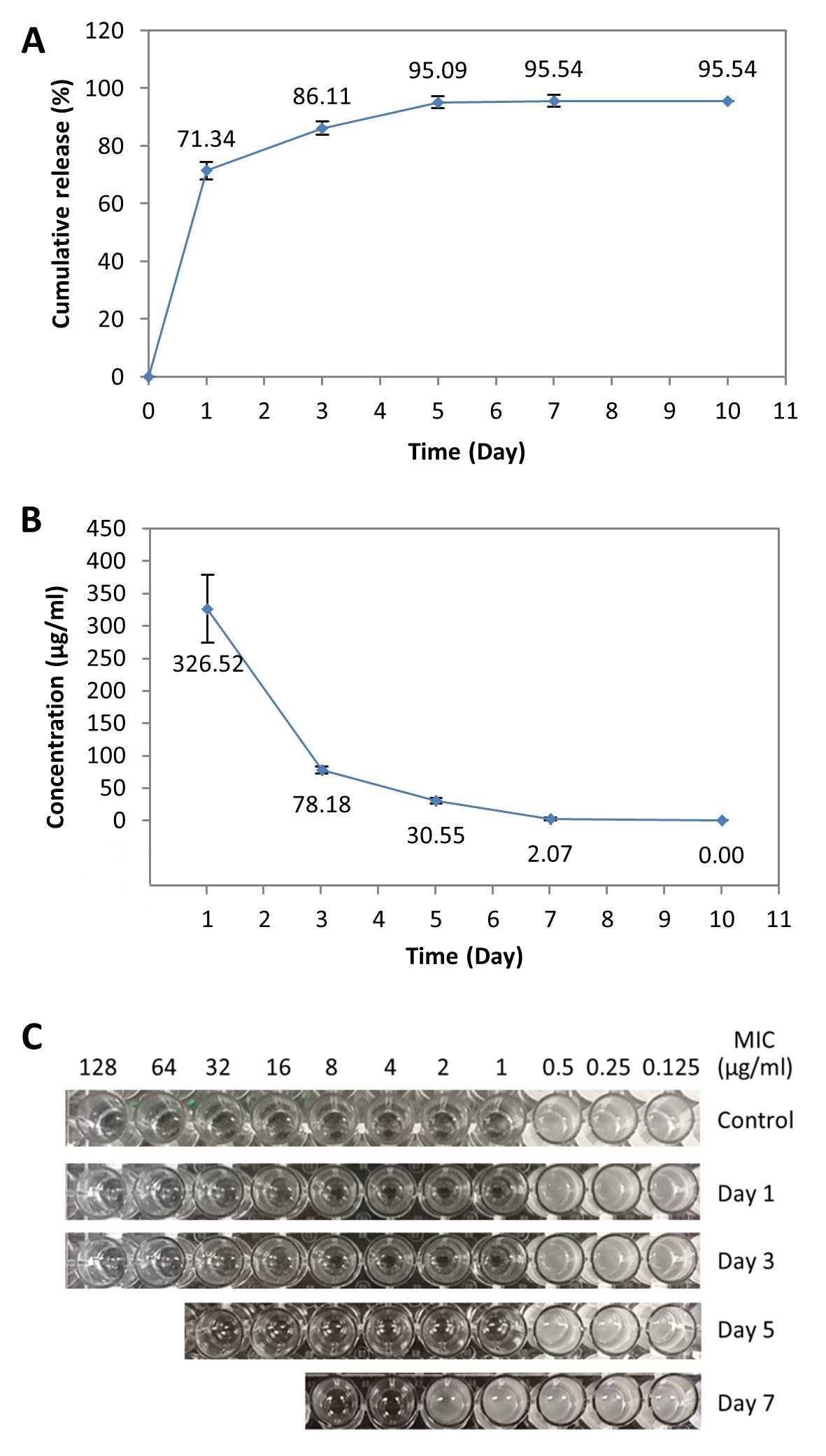
Results and discussion: In vitro, the drug could be released for up to 7 days with concentrations better than theoretical MIC of vancomycin against S. aureus (2 μg/ml) (Figure 1A and B)[3], except the day 7 eluent, which had a reduced MIC (4 μg/ml) (Figure 1C).
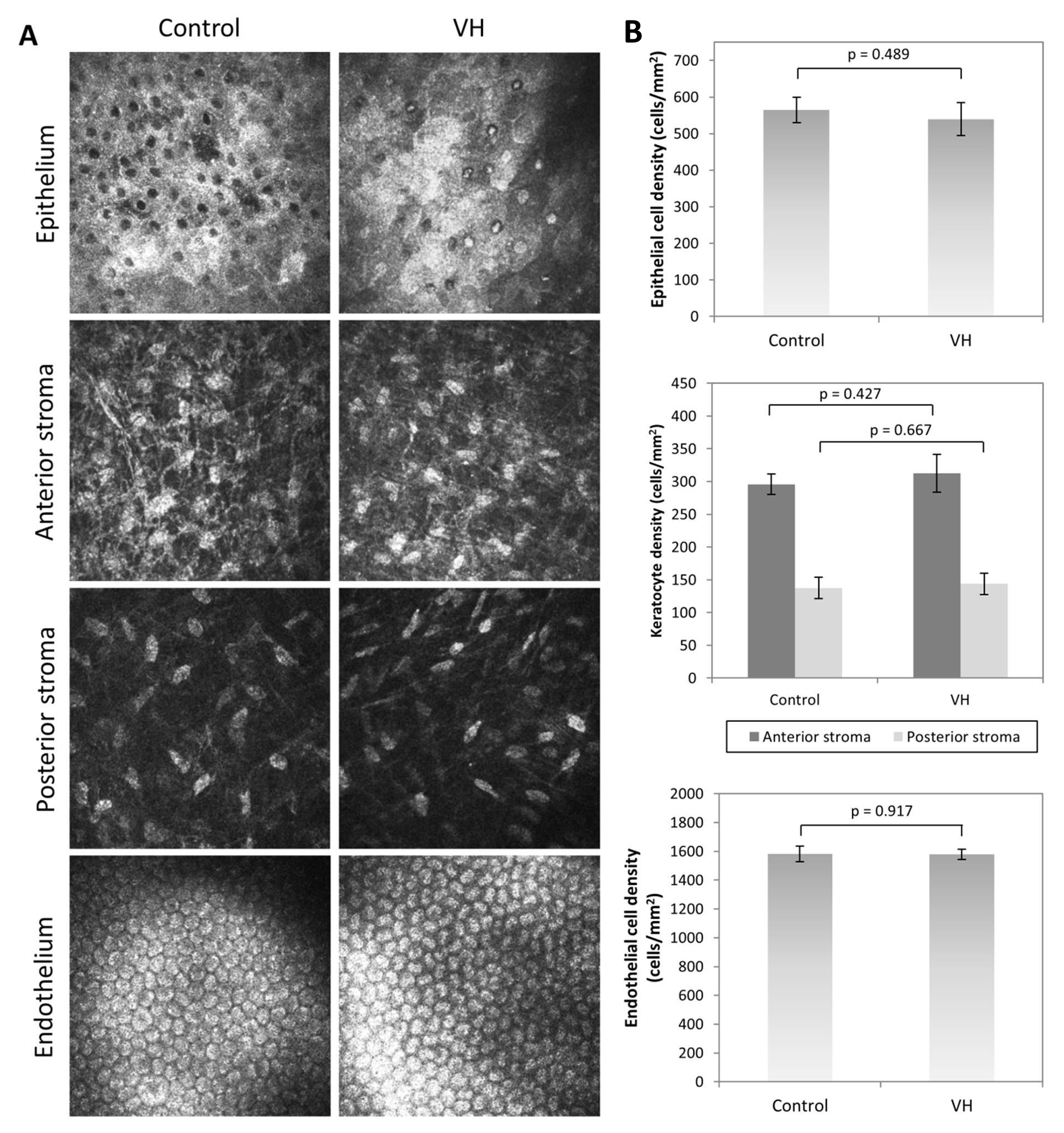
The VH was biocompatible, showing no significant difference (p>0.05) in epithelial, keratocyte, and endothelial cell morphology (Figure 2A) and density (Figure 2B) compared to non-operated corneas after 1 month post-implantation.
On day 3 post-infection (5 days post-implantation), the VH-implanted corneas appeared clear and non-edematous compared to the BH-implanted corneas, which were hazy, edematous, and had excessive inflammation (Figure 3A and B).
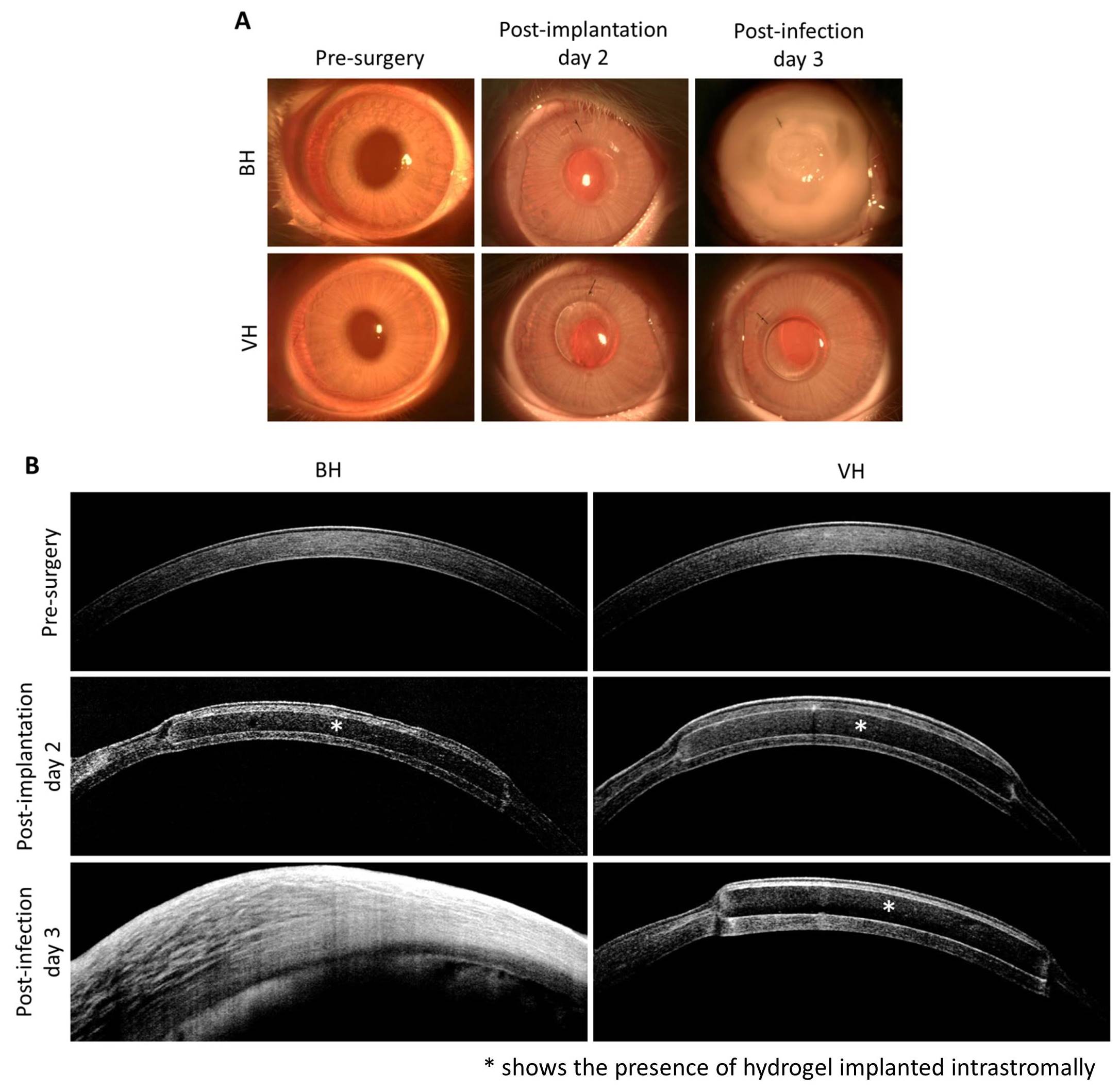
The post-infected corneas were significantly thicker in corneas with BH (713±73 μm) than those with VH (537±56 μm) (p=0.029). Immunohistochemistry further demonstrated a significant reduction in CD18-positive cells in VH-implanted corneas (49±9 cells/unit area) compared to BH-implanted corneas (523±15 cells/unit area) (p<0.001). In addition, there was a log 2.5 reduction in S. aureus in corneas implanted with VH compared to those implanted with BH (p=0.016).
Summary: This study demonstrated the efficacy of localized vancomycin delivery from a collagen-based corneal implant in preventing implantable device-associated S. aureus infections in vivo.
This study was supported by Singapore National Medical Research Council funded Translational and Clinical Research (TCR) Flagship Programme (NMRC/TCR/008-SERI/2013).
References:
[1] Sasaki H, Ichikawa M, Yamamura K, Nishida K, Nakamura J. Ocular membrane permeability of hydrophilic drugs for ocular peptide delivery. J Pharm Pharmacol 1997;49:135-9.
[2] Chew HF, Ayres BD, Hammersmith KM, Rapuano CJ, Laibson PR, Myers JS, Jin YP, Cohen EJ. Boston keratoprosthesis outcomes and complications. Cornea 2009;28:989-96.
[3] Tenover FC, Moellering RC Jr. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis 2007;44:1208-15.