Pluripotent stem cells, such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) are an attractive prospect for tissue engineering and regenerative medicine. The tentative clinical potential of hiPSCs and hESCs is restricted by the use of mouse embryonic fibroblasts (MEFs) as a feeder layer. This is because the possibility of xenogenic contamination during culture restricts the clinical use of transplanted hiPSCs and hESCs. The development of feeder-free cultures using biomaterials having optimal elasticity and nanosegments as cell culture materials will create lower the cost of production without introducing xenogenic contaminants and with more reproducible and reliable culture conditions. Increasing evidence suggests that the physical microenvironments of stem cells, in addition to soluble biological factors, help to direct stem cell fate during proliferation and differentiation[1]. Therefore, we investigated human ESC (H9) and human iPSC culture on biomaterials with different elasticity and grafted with different nanosegments.
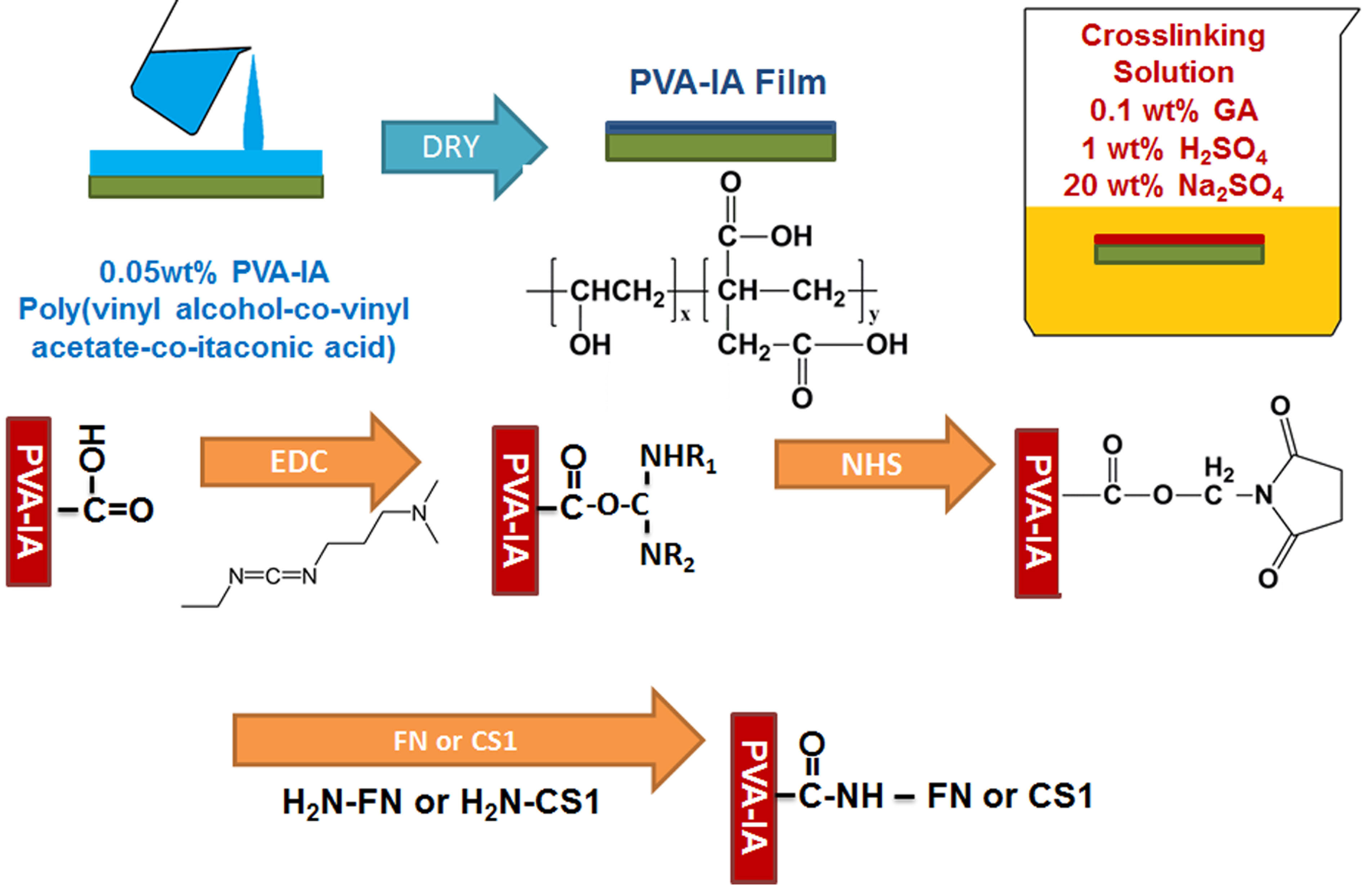
We prepared dishes coated with polyvinylalcohol-co-itaconic acid (PVA-IA) hydrogels having different elasticity ranging from a 3.7 kPa to 30.4 kPa storage modulus by controlling the crosslinking time in crosslinking solution, and grafted with several ECM-derived cell-adhesion peptides. The cell binding domain of vitronectin was grafted onto the PVA-IA substrates. hESCs (H9) and hiPSCs were cultured in a chemically defined medium on several grades of stiffness of PVA-IA hydrogels grafted with oligopeptide derived from vitronectin (KGGPQVTRGDVFTMP). The hESCs and hiPSCs cultured on the most stiff substrates (e.g., storage moduli more than 30 kPa) tended to differentiate after five days of culture, whereas the hESCs and hiPSCs cultured on relatively softer substrates of 12-25 kPa maintained their pluripotency. Only a few small or no colonies of hESCs were observed on the softest substrates (10 kPa).
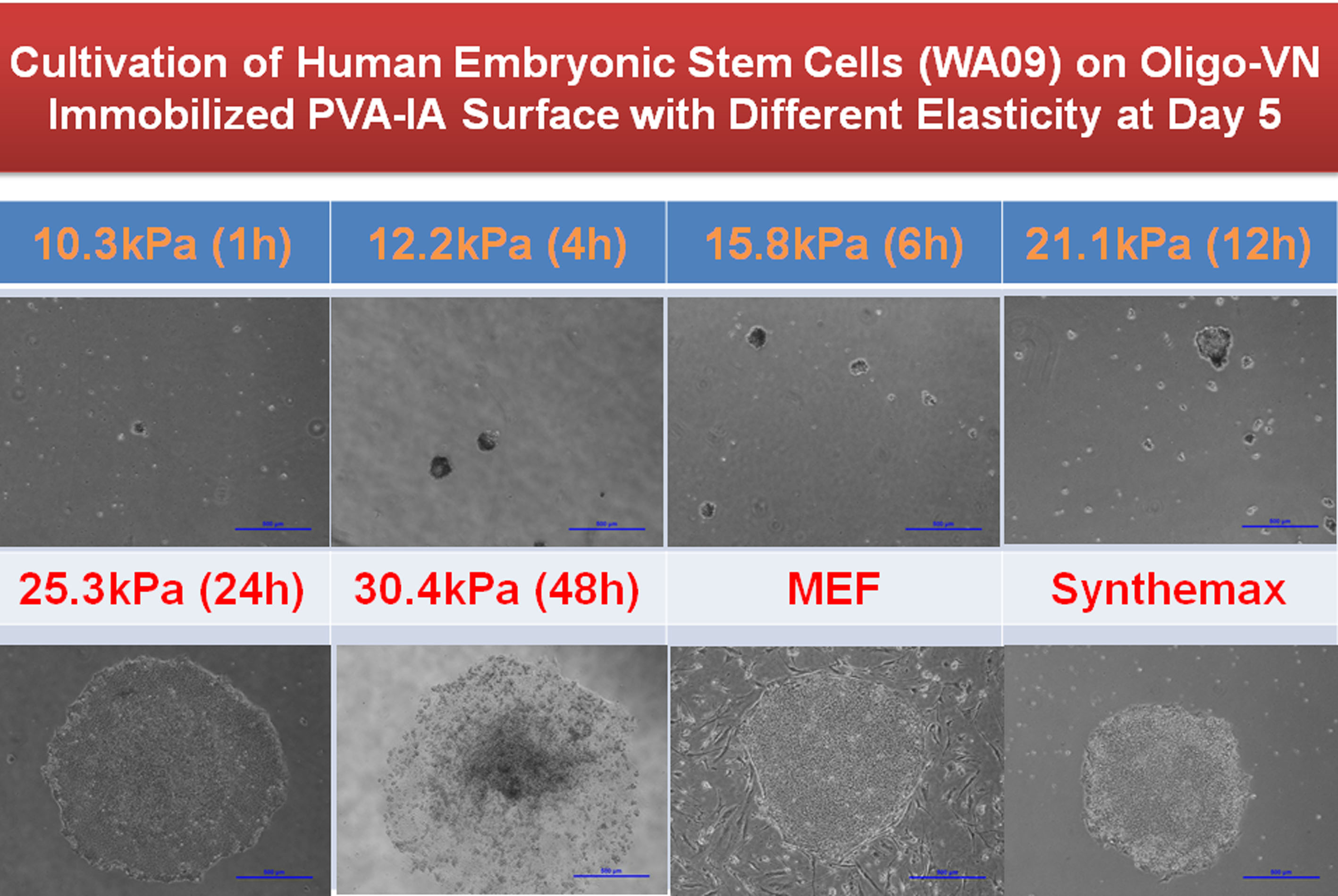
Therefore, these results indicate that cell culture substrates with the optimal elasticity can maintain the pluripotency of hESC and hiPSC culture. It is concluded that both the physical and biological properties of biomaterials affect the ex vivo expansion of HSPCs as well as culture of hESCs and hiPSCs.
Ministry of Education, Culture, Sports, Science, and Technology of Japan (15K06591); Ministry of Science and Technology, Taiwan (103-2120-M-008-001); King Saud University (IHCRG #14-104), Saudi Arabia
References:
[1] A. Higuchi*, Q.-D. Ling, Y. Chang, S.-T. Hsu, A. Umezawa, Chemical Reviews, 113 (2013) 3297-3328.