Introduction: Aseptic loosening of the intramedullary stem in massive segmental bone tumour implants is problematic. We have shown that osteointegration of extracortical bone to a grooved hydroxyapatite (HA) coated collar located adjacent to the transection site reduced implant failure but integration occurred in only 70% of patients[1]. Selective laser sintering (SLS) can produce novel titanium porous components, the inner pores of which can be electrochemically coated with hydroxyapatite (ECHA). We aimed to investigate novel porous collar designs manufactured using SLS and augmented with ECHA. We hypothesised that ECHA coated SLS manufactured collars would enhance osteointegration compared to the current plasma sprayed HA coated grooved design.
Methods: HA coated large pore (Ø750µm, LP), small pore (Ø500µm, SP) and grooved (G) collars were investigated in vivo as part of a diaphyseal tibial implant in an ovine model that remained in situ for 6 months [Fig. 1].
Integration was assessed radiographically and histologically. Extracortical bone (EC) growth was quantified (length, thickness, surface area, % surface contact) between the 3 groups (LP, SP and G) using radiographs. Samples were processed for undecalcified histology and thin sections were prepared through the centre of each collar [Fig. 2].
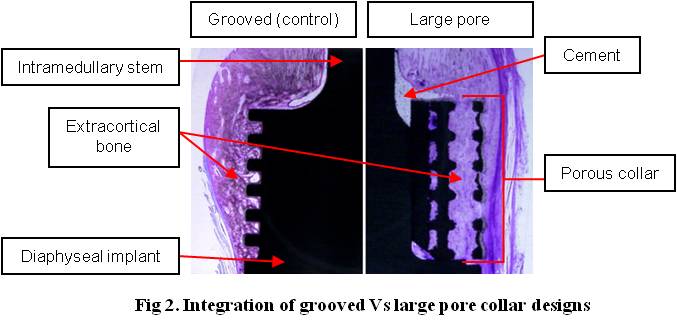
Direct mineralised bony attachment was analysed. A Mann-Whitney U test was used to statistically compare data between groups where p values < 0.05 were considered significant. A Finite Element Analysis (FEA) model was fixed beyond the end of the intramedullary stem with maximum forces recorded during a gait cycle[2] applied to the implant shaft [Fig. 3].
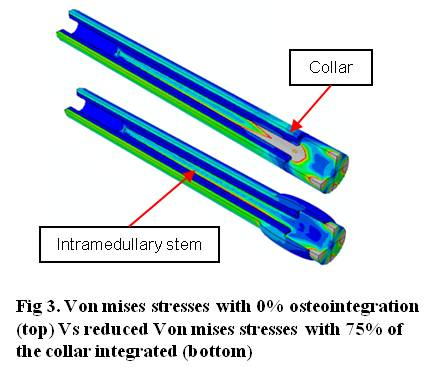
Stress distribution within the bone and implant were investigated at five different levels of osteointegration.
Results: Radiographic analysis revealed a significantly greater EC bone length in G (7.7±4.4mm) Vs LP (4.9±6.5mm, p<0.01) and SP (4.9±6.3mm, p=0.004). Greater thickness of EC growth was seen in G (3.2±1.3mm) Vs LP (1.5±1.7mm, p<0.01) and SP (1.8±2mm, p<0.01). Greater surface area of EC growth in G (15.7±16mm) Vs SP (11.2±18.2mm, p<0.01) and SP Vs LP (10.2±17.4, p=0.031) was found. Both the G (36.4±27.2%, p<0.01) and LP (23.7±31.5%, p=0.031) groups performed significantly better than SP (11.3±28.7%) regarding surface integration of EC bone growth seen radiographically. EC bone attachment to the HA coated surface of the porous collars was confirmed histologically. The surface available for integration (mm, min-max) was significantly greater in SP (226.3, 180.7-262.8) Vs LP (113.4, 59.3-259.5, p<0.01) and G (34.5, 28.2-60.5, p<0.01) as well as LP Vs G(p<0.01). Direct mineralised bony attachment was greatest in SP (56.6, 3.8-180.7, p<0.01) and LP (27.9, 0-106.4, p=0.05) Vs G (13.2, 0-39.8). FEA results indicated that the least amount of integration (25%) caused a reduction in stress removing the risk of implant failure. Loads transmitted within adjacent bone were also reduced in the models where the implant was osteointegrated.
Discussion: Novel porous SLS collar designs combined with ECHA augmented bone growth within the porous structure. Bone ingrowth within pores may provide a stronger mechanism of integration as bone is permeated throughout the collar and not only located on the implant surface. FEA showed that relatively small amounts of osteointegration may be advantageous in reducing high stresses along the intramedullary stem.
Royal College of Surgeons of England; Skeletal Action Cancer Trust; Orthopaedic Research UK
References:
[1] Coathup M et al. Long-term survival of cemented distal femoral endoprostheses with a hydroxyapatite-coated collar: a histological study and a radiographic follow-up. J Bone Joint Surg Am. 2013 Sep 4;95(17):1569-75
[2] Taylor D et al. The prediction of stress fractures using a ‘stressed volume’ concept. J Ortho Res. 2001 Sep;19(5):919-26