Introduction: New Zealand sea urchin, known as kina, shell is a waste material of the seafood industry. There is a significant cost associated with its disposal and waste management. Hydroxyapatite (HA) is a major component of bones and teeth that has been widely used in orthopaedic applications[1]-[3]. HA generated from biogenic sources has high biocompatibility in living organs[4]. In recent years various natural materials, such as egg shells, coral, mussel shells, algae, fish and bovine bones, have been used for the production of HA[5]-[7]. Physical properties, such as phase purity, thermal stability, particle size and shape are important parameters for HA to be employed in biomedical applications[1],[8]. Kina shells skeletal plates are composites of organic and inorganic materials, with regular pores, and tailored structure, which offers unique characteristics for bone grafting. The aim of this study was to develop a porous HA structure from waste kina shell for bone tissue engineering using a hydrothermal process.
Materials and Methods: Cleaned and dried shells were cut to pieces of about 2*2 cm and then heated in a furnace at 700°C to remove organic matter. The pyrolysed samples were then added to a reaction vessel containing 1M of ammonium di-hydrogen phosphate and heated using an oil bath at 100°C with continuous stirring for various time intervals of 1, 2, 4 and 8 days. The mixtures pHs were regulated at 10-11 continuously by a peristatic pump using ammonium hydroxide. At each time interval, samples were removed from solutions, washed with distilled water until neutrality and then dried at 80°C. The physicochemical properties of the obtained HA were characterised using X-ray diffraction (XRD), scanning electron microscopy (SEM), thermal gravimetric analysis (TGA), Fourier transform infrared spectroscopy (FT-IR) and inductively coupled plasma mass spectrometry (ICP-MS).
Result and Discussion: The obtained HA had a porous structure with large pores of 300-400 µm and small pores of 10-20 µm, which is an important characteristic for blood and nutrient circulation and bone regeneration. The crystallization of HA was depended on reaction time and 75% conversion was achieved after 8 days. The HA samples had 4.5 ± 0.04 % of magnesium on wt basis, which is an important component for HA used in bone grafting[9]. The non-toxicity and biocompatibility nature of the samples was confirmed using Saos-2 cell lines through cell cytotoxicity test.
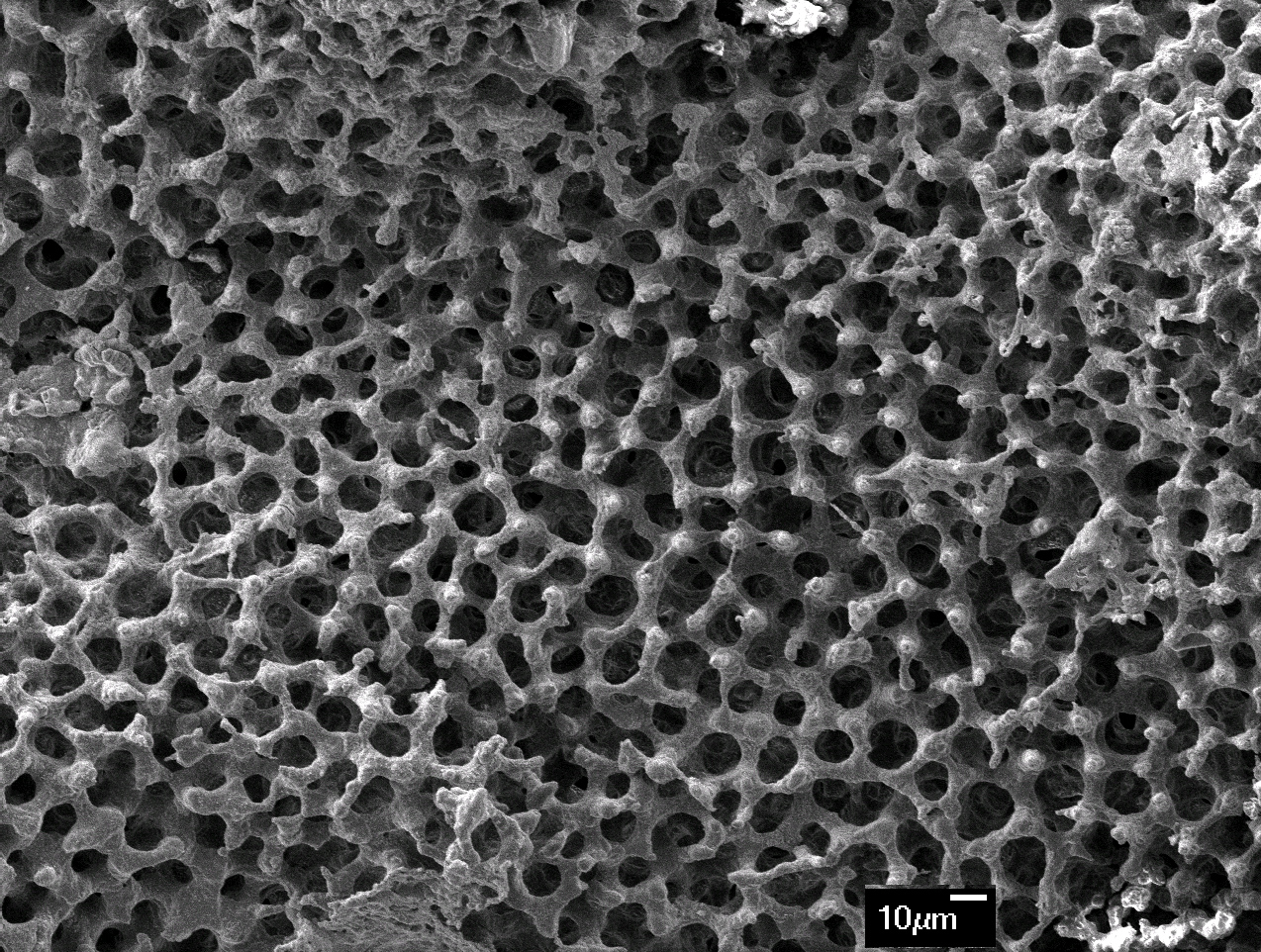
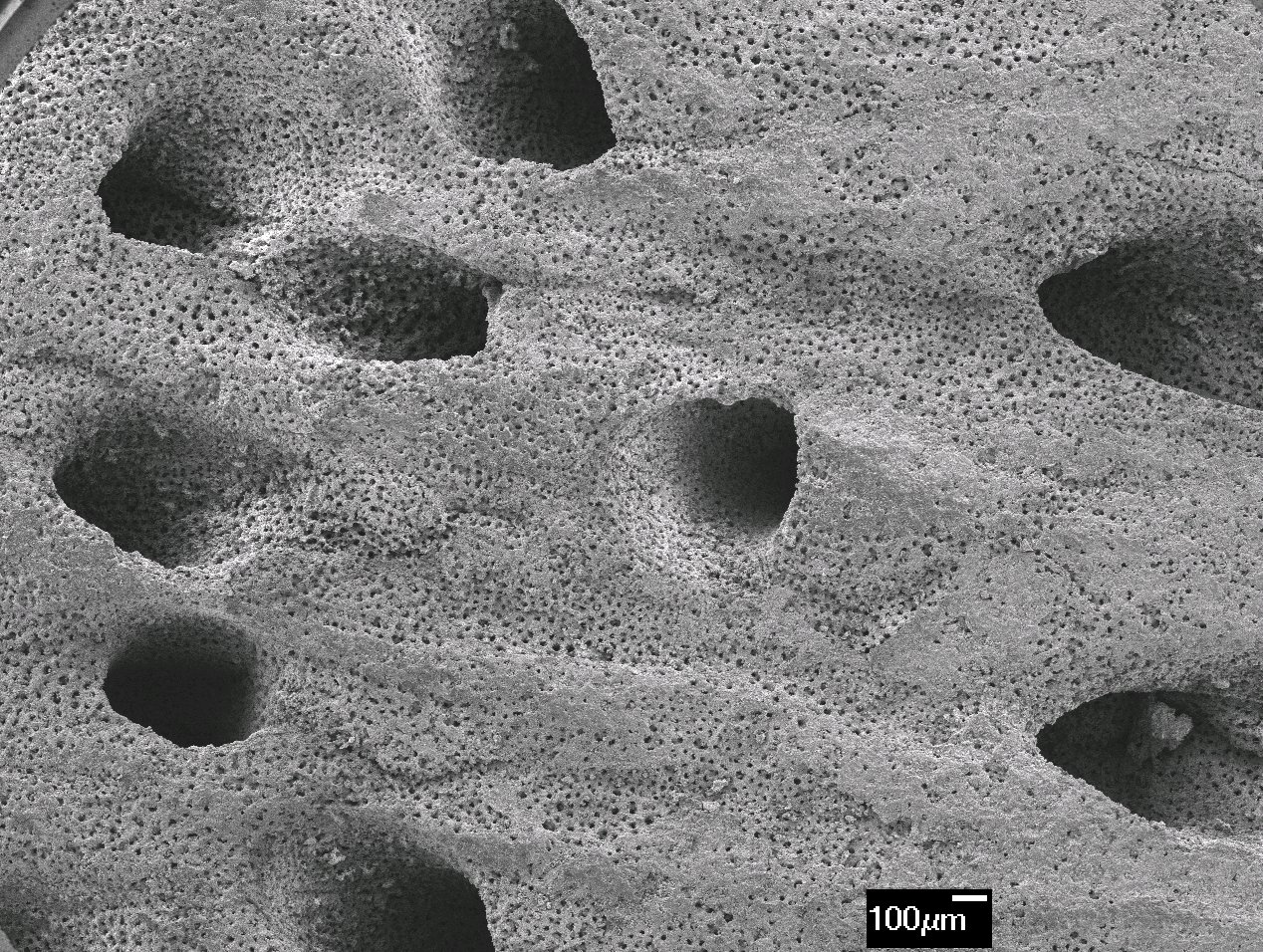
Conclusion: Kina shells were partially converted to Mg-HA with Mg concentration of 4.5 ± 0.04 wt% through a hydrothermal process at 100°C and at atmospheric pressure. The optimum temperature and time for pyrolysis was determined using TGA. The porous and interconnected microstructure of the kina shell was preserved during the reaction. XRD results indicated that HA layer was formed on the surface of the calcium carbonate structure of the shells. Waste kina shells have the potential to enhance bone regeneration and this will be confirmed in in vivo trials.
References:
[1] Akram M, Ahmed R, Shakir I, Ibrahim W, Hussain R. Extracting hydroxyapatite and its precursors from natural resources. J Mater Sci 2014;49:1461-75.
[2] Gao Y, Cao W-L, Wang X-Y, Gong Y-D, Tian J-M, Zhao N-M, et al. Characterization and osteoblast-like cell compatibility of porous scaffolds: bovine hydroxyapatite and novel hydroxyapatite artificial bone. J Mater Sci Mater Med 2006;17:815-23.
[3] Huber F-X, Berger I, McArthur N, Huber C, Kock H-P, Hillmeier J, et al. Evaluation of a novel nanocrystalline hydroxyapatite paste and a solid hydroxyapatite ceramic for the treatment of critical size bone defects (CSD) in rabbits. J Mater Sci Mater Med 2008;19:33-8.
[4] Ripamonti U, Crooks J, Khoali L, Roden L. The induction of bone formation by coral-derived calcium carbonate/hydroxyapatite constructs. Biomaterials 2009;30:1428-39.
[5] Elizondo-Villarreal N, Martínez-de-la-Cruz A, Guerra RO, Gómez-Ortega JL, Torres-Martínez LM, Castaño VM. Biomaterials from Agricultural Waste: Eggshell-based Hydroxyapatite. Water, Air, Soil Pollut 2012;223:3643-6.
[6] Wu S-C, Hsu H-C, Wu Y-N, Ho W-F. Hydroxyapatite synthesized from oyster shell powders by ball milling and heat treatment. Materials Charact 2011;62:1180-7.
[7] Chakraborty R, RoyChowdhury D. Fish bone derived natural hydroxyapatite-supported copper acid catalyst: Taguchi optimization of semibatch oleic acid esterification. Chemical Eng J 2013;215–216:491-9.
[8] Roohani-Esfahani S-I, Nouri-Khorasani S, Lu Z, Appleyard R, Zreiqat H. The influence hydroxyapatite nanoparticle shape and size on the properties of biphasic calcium phosphate scaffolds coated with hydroxyapatite–PCL composites. Biomaterials 2010;31:5498-509.
[9] Suchanek WL, Byrappa K, Shuk P, Riman RE, Janas VF, TenHuisen KS. Preparation of magnesium-substituted hydroxyapatite powders by the mechanochemical–hydrothermal method. Biomaterials 2004;25:4647-57.