Introduction: Stem cell therapy holds promising potential for cartilage repair and regeneration[1]. Controlled delivery of chondrogenic growth factors and small molecues such as TGF (transforming growth factors) and dexamethasone (Dex) is essential to the success of stem cell therapy for cartilage repair and regeneration. Herein, we develop a multifunctional polymersome[2] that is capable of in situ grafting to cartilage for the co-delivery and sustained release of TGF-β3 and Dex to enhance chondrogenic differentiation of hMSCs.
Materials and Methods: Polycaprolactone with hydroxyl terminal group (PCL-OH, 5k) was reacted with methylacryloyl chloride and thiolated β-cyclodextrin (βCD-SH) to obtain PCL-βCD (Fig. 1AB). The BSA-FITC and Rhodamine B base were dissloved in distilled water and DMF (H2o:DMF = 2:1, v/v), respectively, and co-loaded into the PCL-βCD polymersome during the self-assembly process. Hydrogels were generated by polymerizing 2.0% methacrylated hyaluronic acid modified with β-CD (MeHA-βCD) and incubated in the drug-loaded polymersome and linker (ADA-PEG4k-ADA) solution for overnight. (Fig. 2A) Then the obtained hydrogels containing immobilized polymersomes were incubated in 1 ml of PBS or 5mg/ml NH3Cl-ADA solution, respectively (Fig. 2B).
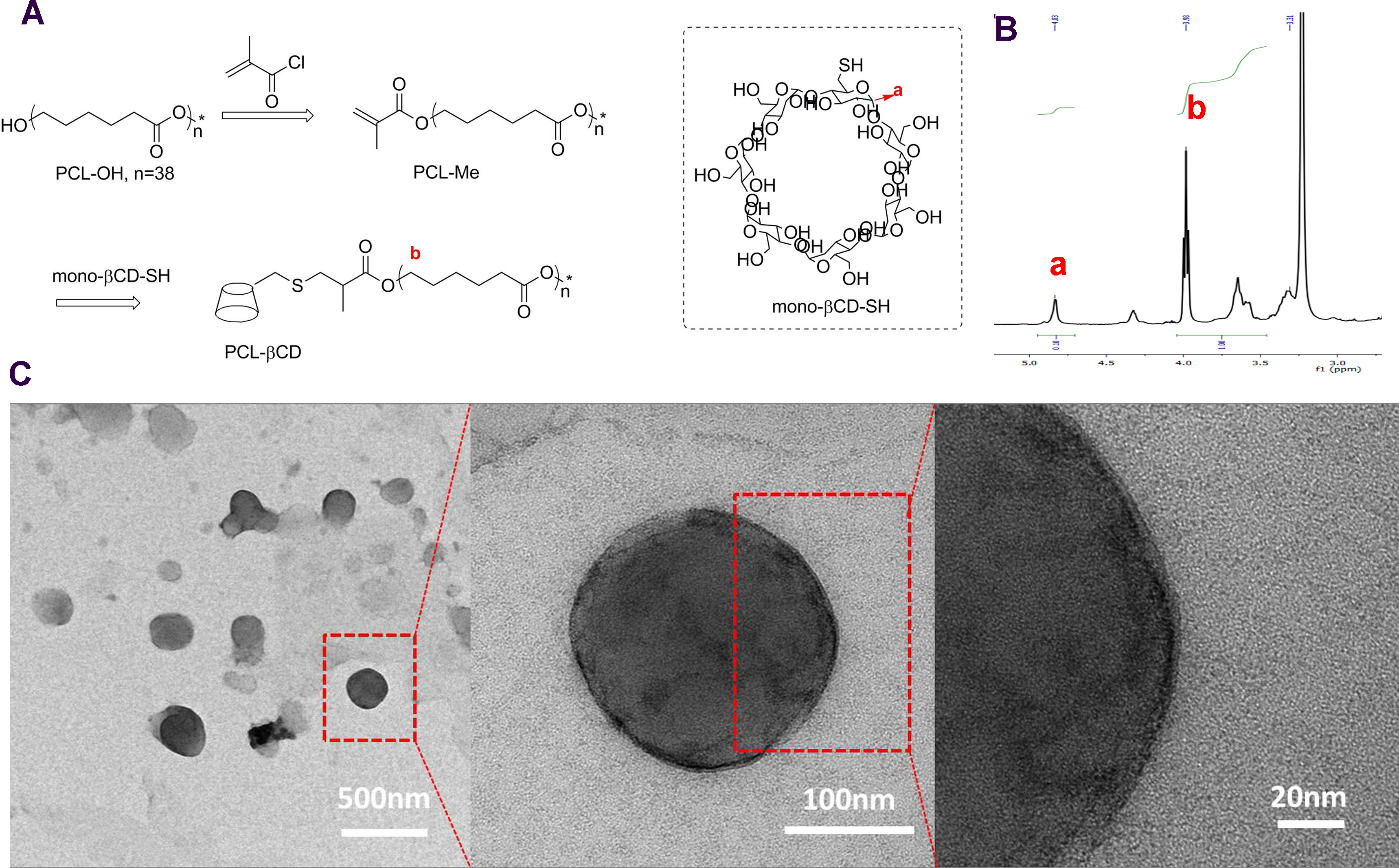
Figure 1. Synthesis of PCL-βCD. (A) synthesis route of PCL-βCD and structure of βCD-SH. (B) 1H NMR of the PCL-βCD. (C) TEM images of self-assembled PCL-βCD polymersomes.
Results and Discussion: The Rh of polymersome (PCL-βCD) is 144nm, and the Rg/Rh is 1.09 based on the dynamic light scattering (DLS) (Rh: hydrodynamic radius, Rg: radius of gyration). This finding supports the assumption (the theoretical value of Rg/Rh = 1) that the scattering objects are vesicles. The vesicle structure is further confirmed by the TEM images, which show the dark rims of the vesicles due to the self-assembled bilayer membrane (Fig. 1C). In both the FITC and Rhodamine channels of the microscope, the HA hydrogels exhibit substantial fluorescence signal, indicating the successful agents loading and immobilization of the polymersomes. In contrast to the hydrogels incubated in PBS, the fluorescence in the hydrogels sharlply decreases after incubation with the competitive free diffusing ADA, which disrupts the host-guest tethering of the polymersome to the hydrogel structure, leading to the release of the polymersomes. This finding shows the successful immoblization of polymeric vesicles into hydrogels via the physical interaction of β-CD and ADA molecules and triggered release of the polymersome and the loaded drugs (Fig. 2C). Significant immobilization of polymersomes on cartilage surface is likely via interacctions between β-CDs of the polymersomes and aromatic groups of the cartilage (Fig. 2 DE).
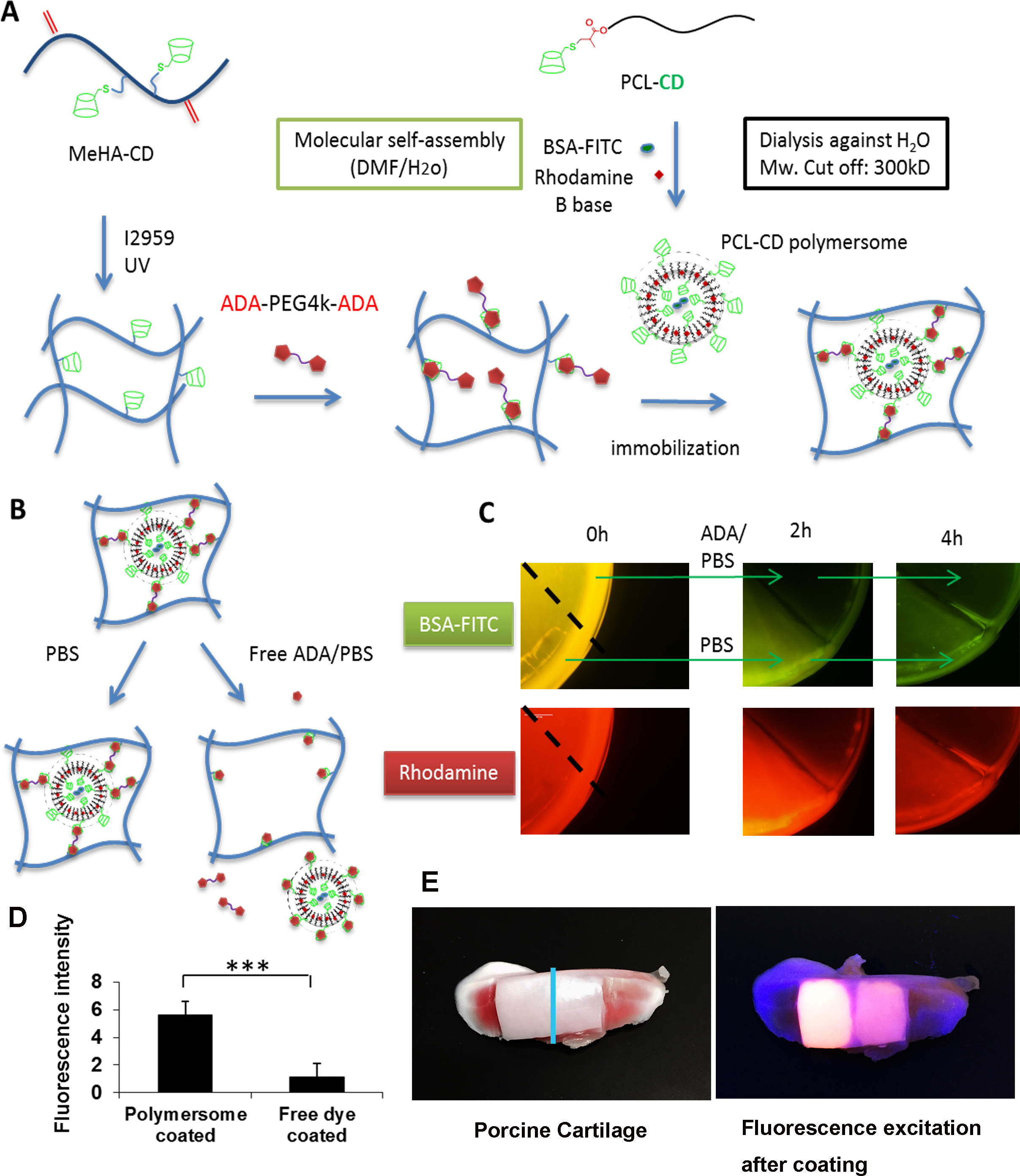
Figure 2. (A) BSA-FITC and rhodamine B base are co-loaded into the PCL-βCD polymersome that are further immobilized in the MeHA-βCD hydrogels. (B-C) Addition of competitive ADA triggers the release of the polymersome and the loaded agents from the hydrogels (dotted line: cutting line of the hydrogel). (D) Quantitative fluorescence analysis of the cartilage coated with polymersomes that are loaded with BSA-FITC and Rhodamine B base (cartilage are treated in the collagenase II for overnight to mimic degenerated cartilage), with free dye solution as the control (***P<0.001, n = 5). (E) Images of porcine cartilage before and after coating trials (Blue line: the cutting site).
Conclusion: The self-assembly polymersomes in this study demonstrate the successful drugs loading, immoblization in the hydrogels via host-guest interaction, and in situ controlled drug release in response to a specific triggering signal. The study shows the promising potential of this polymersome for the triggered co-delivery of chondrogenic growth factors and small molecules for cartilage repair.
The work described in this paper was supported by an Early Career Scheme grant from the Research Grants Council of HongKong.
References:
[1] Buckwalter JA, Mankin HJ. Articular cartilage: Degeneration and osteoarthritis, repair, regeneration, and transplantation. Aaos Instr Cours Lec 1998, 47: 487-504.
[2] Discher BM, Won YY, Ege DS, Lee JCM, Bates FS, Discher DE, et al. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284(5417):1143-1146.