Cell sheets prepared via gel-sol transition of calcium RGD-Alginate
-
1
Queen's University, Department of Chemical Engineering, Canada
-
2
Queen's University, Human Mobility Research Centre, Canada
Introduction: Cell sheet engineering has emerged as a novel approach for forming layered tissue and shown positive outcomes in clinical tissue engineering applications. With the objective of developing a simple and inexpensive process for the generation of contiguous and viable cell sheets, sodium alginate was first conjugated with the integrin binding peptide sequence RGD and crosslinked with calcium ions. 3T3 fibroblasts and human corneal epithelial cells (HCECs) were seeded onto the gel surface, grown to confluence, and a cell sheet harvested following chelation with sodium citrate.
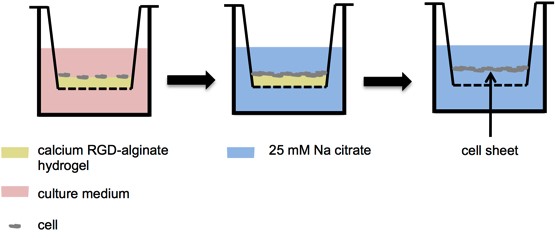
Materials and Methods: The peptide GGGGRGDS was conjugated to sodium alginate utilizing aqueous carbodiimide chemistry, obtaining degrees of substitution of 5 and 13%. 10 mg/mL (100 μL) RGD-alginate solution was added into 12-well inserts, dried to form a thin layer then crosslinked by 2 mL of 10 mM CaCl2 solution for 16 h. 500 μL cell suspensions of HCEC or 3T3 cells at low (6000 cells/cm2) and high density (6.0 × 104 cells/cm2) were seeded onto the surface of the RGD-alginate hydrogels. Cell adhesion and proliferation was monitored by light microscopy. The Live/DeadTM assay was used to evaluate cell viability. The cell sheet was harvested by adding 500 μL of 25 mM sodium citrate into the insert and incubating at 37 ºC for 20 min (Figure 1). Cell-cell junction retention before and after sodium citrate treatment were compared via the extent of immunostaining using rabbit monoclonal anti-E cadherin and anti-N-cadherin antibody for cell sheets of HCECs and 3T3s. To demonstrate the possibility of forming stacked cell layers, HCEC sheets were stained with CellTracker of different colors, grown on top of each other to form a multilayer tissue, and imaged using a confocal microscope.
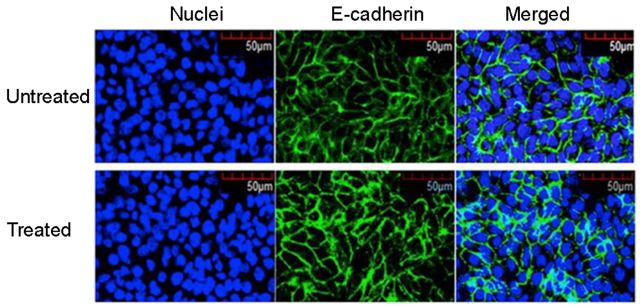
Results and Discussion: RGD significantly improved attachment of both 3T3 cells and HCECs, while very poor cell attachment was observed on the surface of pristine calcium alginate. Cell sheets were readily harvested for both cell types. A potential concern with this approach was the possibility of disruption of cell-cell adhesion within the cell sheets following sodium citrate treatment. For HCECs, E-cadherin is responsible for maintaining the structural integrity of the epithelial layer. By comparison of the extent of staining of E-cadherin before and after citration via immunostaining (Fig. 2), no apparent disruption of calcium bound to E-cadherin was observed because of the lower binding affinity of the calcium ion for the guluronic acid sequences in alginate than for the cadherin calcium binding sites. Moreover, the possible presence of residual alginate on the cell sheet formed with HCECs did not prevent stacking of these cell sheets to form multilayer tissues.
Conclusion: An inexpensive approach to harvesting cell sheets using calcium alginate was developed capable of forming multilayer cell sheets that retain cell-cell adhesion.
Funding for this project was provided in part by the Natural Sciences and Engineering Research Council of Canada CREATE Biointerfaces Training Program and the 20/20 Ophthalmic Biomaterials Network.
Keywords:
Intelligent gel,
bioactive interface,
matrix-cell interaction,
cell sheeting
Conference:
10th World Biomaterials Congress, Montréal, Canada, 17 May - 22 May, 2016.
Presentation Type:
Poster
Topic:
Protein interactions with biomaterials
Citation:
Yan
J,
Chen
F and
Amsden
BG
(2016). Cell sheets prepared via gel-sol transition of calcium RGD-Alginate.
Front. Bioeng. Biotechnol.
Conference Abstract:
10th World Biomaterials Congress.
doi: 10.3389/conf.FBIOE.2016.01.01215
Copyright:
The abstracts in this collection have not been subject to any Frontiers peer review or checks, and are not endorsed by Frontiers.
They are made available through the Frontiers publishing platform as a service to conference organizers and presenters.
The copyright in the individual abstracts is owned by the author of each abstract or his/her employer unless otherwise stated.
Each abstract, as well as the collection of abstracts, are published under a Creative Commons CC-BY 4.0 (attribution) licence (https://creativecommons.org/licenses/by/4.0/) and may thus be reproduced, translated, adapted and be the subject of derivative works provided the authors and Frontiers are attributed.
For Frontiers’ terms and conditions please see https://www.frontiersin.org/legal/terms-and-conditions.
Received:
27 Mar 2016;
Published Online:
30 Mar 2016.
*
Correspondence:
Dr. Jing Yan, Queen's University, Department of Chemical Engineering, Kingston, ON, Canada, shui.wu.you@163.com
Dr. Fei Chen, Queen's University, Department of Chemical Engineering, Kingston, ON, Canada, Email1
Dr. Brian G Amsden, Queen's University, Department of Chemical Engineering, Kingston, ON, Canada, brian.amsden@queensu.ca