Nanomedicine is developing new strategies to face old and new, not-yet fully understood pathologies. New drugs, surface implants and imaging techniques are based on the properties of nanoparticles. For in-vivo applications, checking their short- and long-term biocompatibility is mandatory. Aim of this study was to investigate the direct biointeraction of submicron- and nano-sized particle in the blood of patients affected by leukemia, who were exposed to particulate matter.
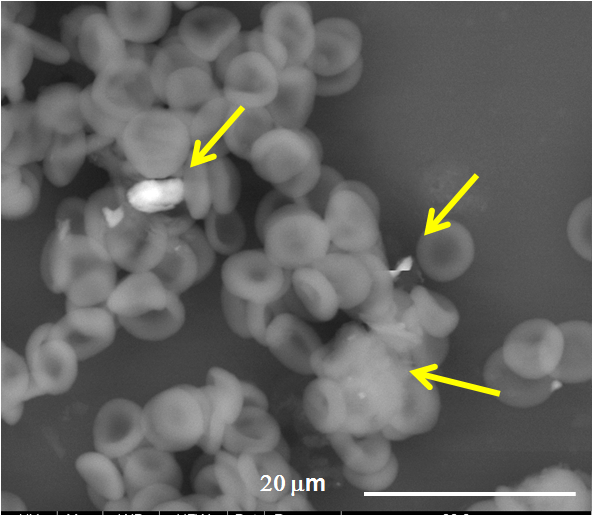
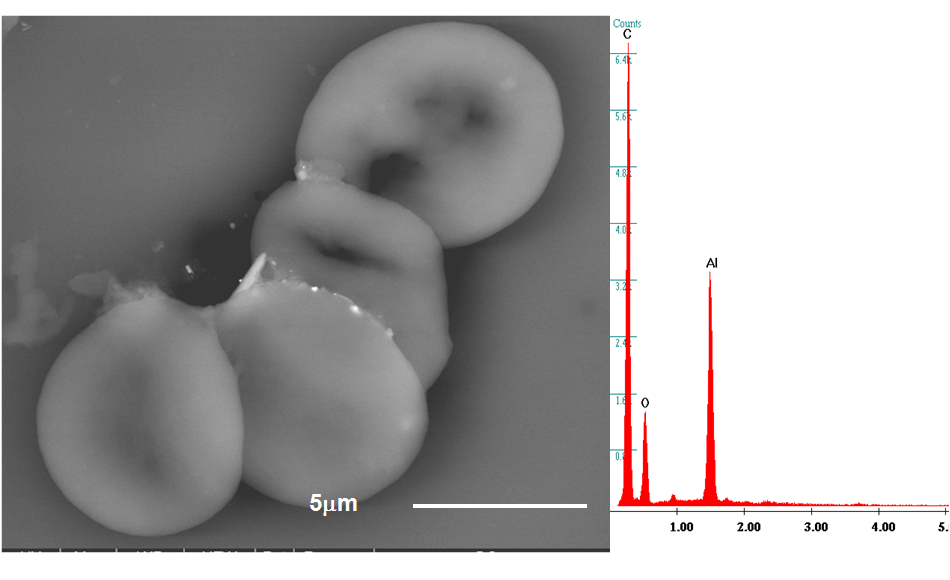
38 peripheral blood samples acute-myeloid leukemia cases (AML), 19 healthy controls] were analyzed by means of a Field-Emission-Gun Scanning Electron Microscope (Quanta 250, The Netherlands) coupled with an Energy Dispersive Spectroscope (EDAX, USA), to identify composition and morphology of micro- and nano-sized foreign bodies present in those samples. After fixation in glutaraldehyde, the samples were centrifuged and the fractions analyzed through Flow Cytometry (BD FACSCalibur™, USA). Three distinct fractions were obtained: plasma, middle layer with white cells, and red cells. In every fraction foreign bodies (ranging < 100 nm - 10 µm) attached singularly to the cells or dispersed in aggregated forms in the plasma were identified. Their chemical composition was evaluated and the frequency of every element counted. Unpaired two-tailed Student’s t-test, MANOVA and Principal Component Analysis were applied to the results. They showed that the total numbers of aggregates and particles proved to be statistically different between cases and controls (MANOVA, P<0.001 and P=0.009 respectively). The aggregates resulted rich in heavy metals such as iron, chromium, nickel (i.e. the alloy of stainless steel), titanium and lead. All the elements were more frequent in pathological cases as compared to controls. Si, Al, Fe, Ti, and Cu were significantly more frequent in AML (P=0.03, P=0.03, P=0.002, P=0.04, P=0.02 respectively). Ni (101vs5), Cr (142vs65), and Pb (57vs2) particles were clearly the small sample size. Also, Principal-Component-Analysis clearly pointed out a strong difference between cases and controls.
The particulate matter (engineered or nanosized by-products) identified in the blood has probably an environmental origin, but some chemical compositions may belong to nanotechnological-surfaced implants. Their presence in the blood induced a nanobiointeraction (protein-corona effect). These organic-inorganic compounds can be the start of a thrombus and of other pathologies.
The technique reported can detect possible releases of nanoparticles in the blood from nanotechnological implants or imaging technique and it can work to verify their biocompatibility. Further it shows a higher presence of particles in AML patients than in controls.
References:
[1] Gatti AM, Montanari S. Case Studies in Nanotoxicology and Particle Toxicology, 1st Edition. Academic Press, Elsevier (USA); 2015, 1-260.
[2] Tenzer S, Docter D, Rosfa S, Wlodarski A, Kuharev J, Rekik A, Knauer SK, Bantz C, Nawroth T, Bier C, Sirirattanapan J, Mann W, Treuel L, Zellner R, Maskos M, Schild H, Stauber RH. Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: a comprehensive quantitative proteomic analysis. ACS Nano. 2011 Sep 27;5(9):7155-67.