Introduction: A severely impaired esophagus requires a largely invasive standard of care surgery known as an esophagectomy, whereby the surgeon removes the affected tissue of the esophagus and splices the stomach or intestine on to the remaining tissue [1]. This results in high morbidity rates and a low quality of life [2]-[5]. Our solution for esophageal resection, is to develop a decellularized synthetic electrospun tubular scaffold from absorbable polymers such as polycaprolactone (PCL) and chitosan (CS) loaded with established therapeutic factors from anti-inflammatory allogeneic bone marrow derived human mesenchymal stem cells (MSC2s) [6] as an “off-the shelf” implantable device that can robustly regenerate a damaged portion of an esophagus.
Materials and Methods:
1) Electrospinning: Prepare a concentration solution of CS:PCL at 10% (w/v). Dispense the solution onto a rotating stainless steel rod through a 20 gauge blunt needle. Sterilize the tubular scaffold in 70% ethanol for 30 minutes. Wash 3X with sterile deionized water and freeze-dry.
2) Cell Culture of MSCs on PCL-CS: Seed the PCL-CS scaffold with MSCs at 600,000/cm2 in a sterile polypropylene cell culture tube. Culture MSCs on the PCL-CS scaffold for 2-3 days in the polypropylene tube within a humidified incubator at 37oC and 5% CO2.
3) Freeze/thaw Decellularization: Freeze the MSC seeded PCL-CS construct in deionized water at -80oC overnight. Afterwards, thaw the construct at room temperature and immediately place the construct in liquid nitrogen for 10 minutes followed by an immediate immersion in a 37oC water bath for 10 minutes. Repeat 2X.
4) Scanning Electron Microscopy (SEM): Fix samples with 10% formalin and then dehydrate in a graded ethanol (EtOH) series. Mount on SEM and sputter coat.
5) DAPI Staining: Fix samples with 10% formalin and incubate in a 30 uM DAPI solution for 10 minutes at room temperature. Wash samples with phosphate buffer saline (PBS) and record fluorescent images.
Results and Discussion: An effective improved approach with the device is to utilize the MSC2’s potent and predictive anti-inflammatory therapeutic factors to provide robust wound healing of a damaged esophagus. In order to obtain the necessary secreted factors on the PCL-CS scaffold, first MSCs need to viably adhere and grow on the material [Figure 1].
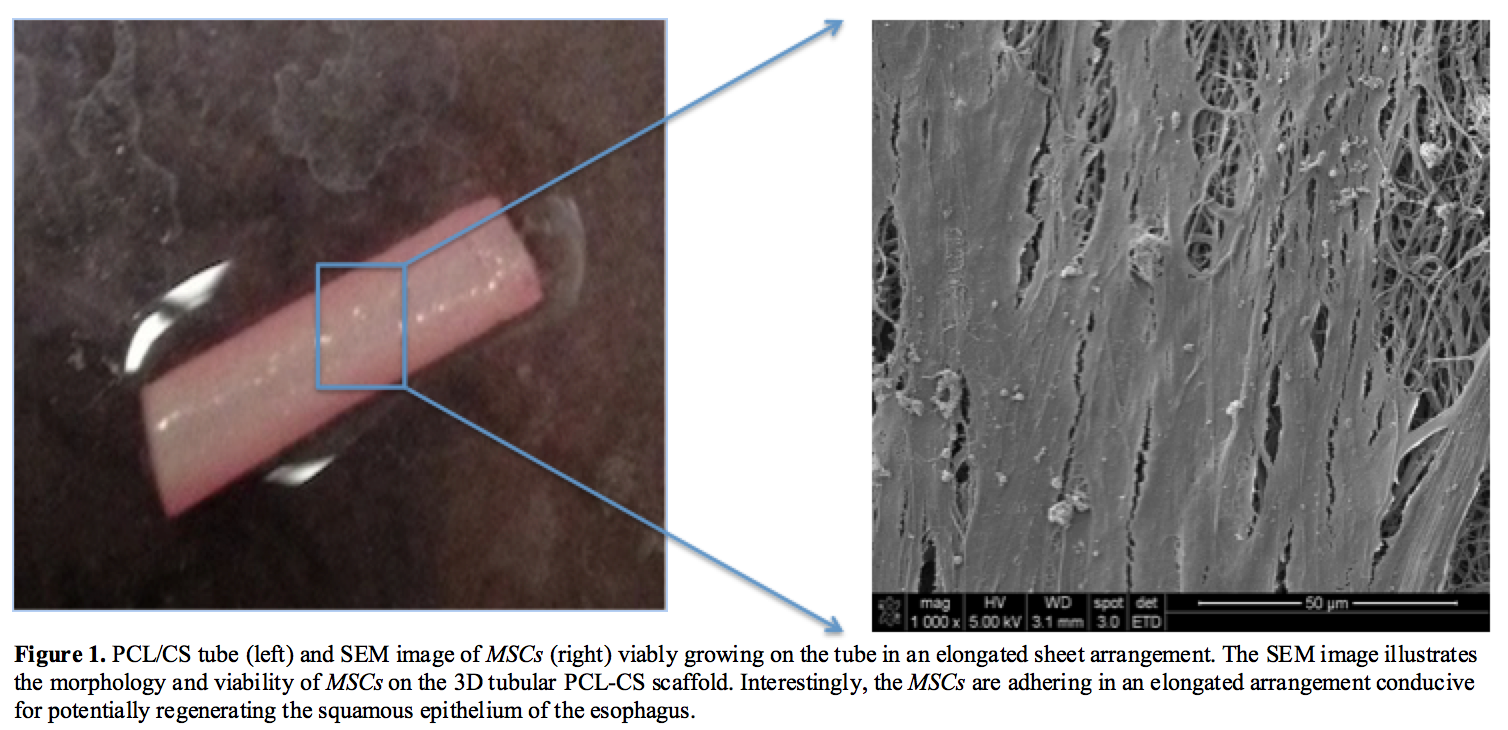
The PCL-CS MSC2 seeded construct is decellularized to develop an implantable “off-the-shelf” absorbable device for potential use by thoracic surgeons to repair a damaged esophagus. To avoid residual detergents that are traditionally used to decellularize tissues, a freeze/thaw decellularization strategy is suggested and assessed here [Figure 2]. Overall the freeze/thaw method established is properly decellularizing the PCL-CS construct.

Conclusion: MSCs can be viably seeded on a tubular PCL-CS scaffold and subsequently this construct can be decellularized. Electrospinning the PCL-CS tube allows for a specific biomechanical design to not only allow MSC2s to properly adhere, but potentially allow native esophageal precursor cells to infiltrate the scaffold and robustly regenerate the esophagus [7],[8]. The freeze/thaw decellularization method provides an ideal translational medical device that can be packaged in saline solution for up to 1 year. This research initiates a path in developing a synthetic construct to be used by a thoracic surgeon as a one time use device implant to robustly regenerate an impaired esophagus.
Funding was provided by the National Institutes of Health 1P20RR20152-01, 1R43AR061902, Department of Defense OC073102 Concept Award, National Science Foundation IGERT Award 1144646. Research support from the Tulane Cancer Center and the Center for Stem Cell Research and Regenerative Medicine, and Histogen, Inc.
References:
[1] Kerm, C. S., Leong, M. F. & Kono, K., Regenerative medicine for oesophageal reconstruction after cancer treatment. Lancet Oncol 16, e84-92 (2015).
[2] Hur, C. et al., Development, Calibration, and Validation of a U.S. White Male Population-Based Simulation Model of Esophageal Adenocarcinoma. PLoS ONE 5 (3), e9483. doi:10.1371/journal.pone.0009483 (2010).
[3] Gordon, L. G. et al., Modeling the Cost-effectiveness of Strategies for Treating Esophageal Adenocarcinoma and High-grade Dysplasia. J Gastrointest Surg 16, 1451–1461 (2012).
[4] Pinheiro , P. F. M., Simões e Silva , & Pereira , , Current knowledge on esophageal atresia. World J Gastroenterol 18 (28), 3662-3672 (2012).
[5] Lupa, M., Magne, , Guarisco , J. & Amedee , R., Update on the Diagnosis and Treatment of Caustic Ingestion. The Ochsner Journal 9, 54-59 (2009).
[6] Waterman , R. S., Tomchuck, S. L., Henkle, S. L. & Betancourt, A. M., A New Mesenchymal Stem Cell (MSC) Paradigm: Polarization into a Pro-Inflammatory MSC1 or an Immunosuppressive MSC2 Phenotype. PLoS ONE 5 (4), e10088. doi:10.1371/journal.pone.0010088 (2010).
[7] Lv , , Chen, L., Zhu, Y., Hou, L. & Liu, Y., Promoting Epithelium Regeneration for Esophageal Tissue Engineering through Basement Membrane Reconstitution. ACS Appl. Mater. Interfaces 6, 4954−4964 (2014).
[8] Iyer, S., Udpa, N. & Gao, Y., Chitosan selectively promotes adhesion of myoblasts over fibroblasts. J Biomed Mater Res , 00A:000–000 (2014).