Introduction: 3D printing has emerged as a promising forefront for complex tissue engineering due to advanced design capabilities. However, a severe limitation to its growth is the lack of bioinks and the inability to tune mechanical, biological, chemical, and physical properties of the material without compromising “printability”[1]. We have developed a single bioink method capable of producing extrudable, soft hydrogels of various formulations utilizing PEG cross-linkers (PEGX). We have manipulated concentration, material type, and degree of cross-linking in hydrogels of amine-containing polymers cross-linked with bifunctional PEG-NHS[2]. Here, we probe the chemical versatility of the method as well as what PEGX polymer properties (molecular weight, architecture) can generate tunable, 3D printable hydrogels.
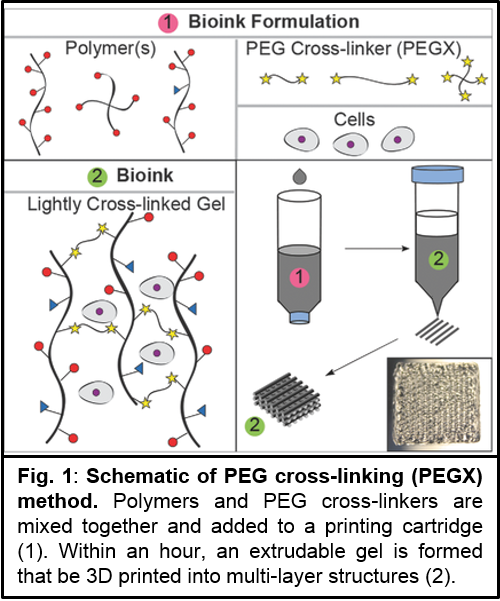
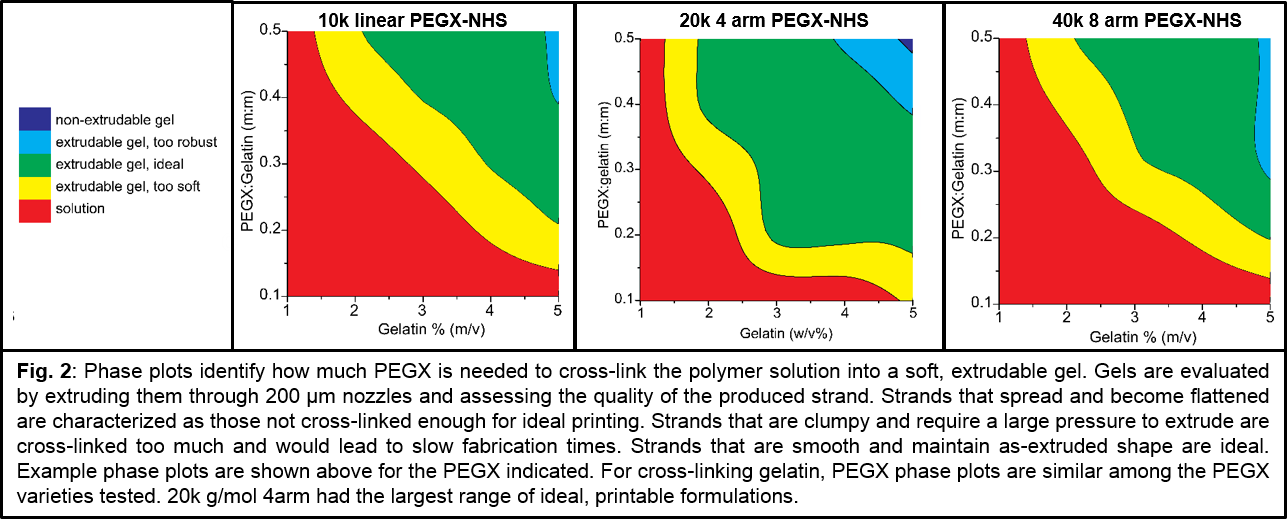
Methods and Materials: Inks are synthesized by mixing polymer and PEGX with or without cells and allowing the mixture to gel within a printing cartridge at 37 °C (Fig.1). A variety of PEGX was investigated: NHS – linear (5k, 10k, 20k g/mol), 4 arm (10k, 20k g/mol), 8 arm (40k g/mol) to cross-link gelatin and PEG-maleimide, acrylate, and vinyl sulfone to cross-link PEG-thiol. To determine printable formulations, phase plots were created by screening varying polymer concentrations with varying PEGX:polymer mass to mass (m:m) ratios (Fig.2). Material “phase“ (i.e. solution, gel) was determined by tube inversion, and extrusion of gels through a 200 µm tip was attempted. Human dermal fibroblasts were encapsulated, and extruded gels were imaged with confocal microscopy and Live/dead stain.
Results and Discussion: For gelatin inks, we found that nearly all studied PEGX varieties led to printable gels. Only 20k linear PEGX did not produce any printable gels at the mass ratios studied. All other cross-linkers displayed a similar “window“ of printability, with the 20k 4arm PEGX producing the largest printable range (Fig.2). The fact that polymer architecture can be manipulated to use multiarm PEG suggests that heterofunctional PEGX may be utilized for adding imaging, sensing, delivery or bioactive modalities. In our investigations of thiol-based cross-linking, we successfully established that the chemistry can be changed while maintaining printability since all studied chemistries generated soft, extrudable gels. Furthermore, encapsulated cells were viable one day post-printing (Fig.3).
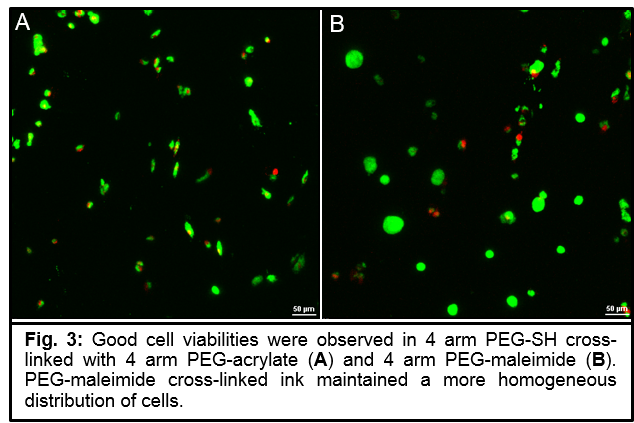
Conclusions: We have developed a bioink method to be utilized for expanding the number of 3D printable and cell-compatible materials. We have validated that the method is independent of a specific cross-linking chemistry and can be used to develop printable materials of various functionalities. Further, we established that different physical varieties of PEGX can be used, including multi-arm PEGX. We hope that this method will contribute towards advancing bioinks to enable biofabrication of compositionally and structurally complex structures and functional tissues.
References:
[1] Malda J et al. Adv Mater. 2013;25(36):5011-28
[2] Rutz A et al. Adv Mater. 2015:27(9):1607-1614.