Introduction: Biodegradable metals made of magnesium and its alloys have been investigated as implant materials for more than 100 years[1]. But just recently, first in vivo results confirmed the suitability of open-porous biodegradable scaffolds made from sintered magnesium fibers providing a stochstic distribution of pores[2]. In this context, Additive Manufacturing (AM) technologies enable the realisation of more complex and biomechanically predetermed parts. An interesting AM technology is Selective Laser Melting (SLM) which uses laser radiation to subsequently melt and fuse thin layers of metal powder according to a sliced CAD-model. Thus, SLM has been investigated in this study to manufacture individualised bone implants with highly interconnected porosity.
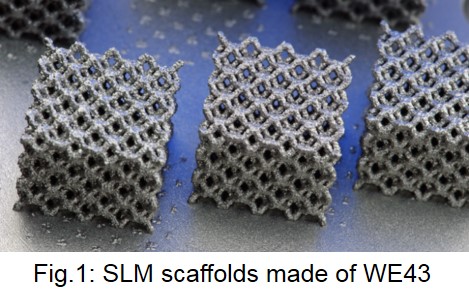
Materials and Methods: A laboratory SLM setup consists of an optical setup using of a single mode ytterbium fiber laser (IPG YLR-200) with 230W maximum output power, a galvanometric scanner (SCANLAB hurrySCAN 20) and a f-theta focussing lens (SILL S4LFT 3254/126). The process chamber enables processing in an argon inert gas atmosphere with oxygen content below 10ppm. The materials used are gas atomized powders out of Mg alloy WE43 (4wt% yttrium, 3wt% rare earths)with almost spherical particle shape (MSE Clausthal, Germany). The powders are sieved to particle sizes 25-63µm before SLM processing. To build various SLM test specimen, the main SLM process parameters such as laser power, scan speed, hatch distance and exposure strategy are varied. SLM test specimen are analysed by means of SEM, EDS and light microscopy. In vitro corrosion test were performed in a perfusion bioreactor under cell culture conditions in HANKS solution by determining the corrosion rate from metallic volume loss analysed by microCT. Additionally biomechanical compresion tests were performed. Cytocompatibility has been tested by viability tests (MTS) with primary human osteoblasts (hOB) using 72h extract testing according to ISO10993-5 .
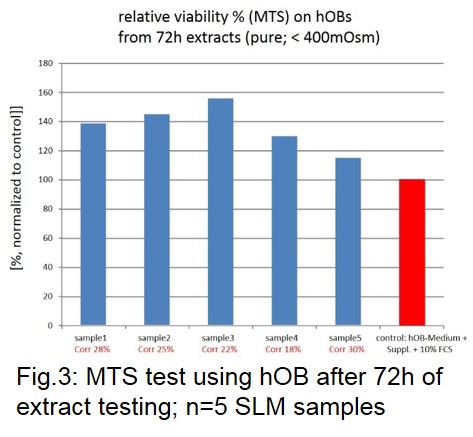
Results: According to EDS analysis of cross-sections of SLM-specimens (Fig.1) the magnesium content of the SLM-specimens is linearly dependent on the energy input per unit volume; with more Mg evatoration at higher the energy. However, the part density is usually higher for higher energy input. The resulting parts show strong sintering of particles (Fig.2 left). However, these adhering particles can be removed by sandblasting (Fig.2 right). Sandblasted parts exhibit almost dense struts with thicknesses down to 300µm. The corrosion tests (n=5) revealed homogenous corrosion with 25% (SD 5%) volume loss over 72h. Extracts tests (n=5) showed high viablity with hOB after 72h, even with undiluted extracts (<400mOsm) (Fig.3). The compression tests (n=6) provided ultimate compressive strength (UCS) in a range between 20-23 MPa.

Discussion: When processing WE43 by SLM at higher energy input the amount of magnesium content in test specimens is reduced but the part density is increased. The fabricate scaffolds with designed interconnected porosity showed suitable corrosion rates, high cytocompatibility and suitable compression tests to replace cancellous bone.
Conclusion: This study shows the feasibility to produce open-porous, biodegradable and biocompatible magnesium scaffolds by SLM. This is a first step towards manufacturing of individualised bone implants with designed, interconnected porosity.
References:
[1] F. Witte, Acta Biomaterialia 2010, 6:1680-1692.
[2] K. Bobe et al., Acta Biomaterialia 2013, 9:8611-8623.