Introduction: Most tissues are composites of several different kinds of extracellular matrix (ECM) components including collagens, elastin, and proteoglycans. Unique composition and organization of ECM allows tissues to serve the various biomechanical needs of the body. Heart valves, in particular, operate in an intense mechanical environment, opening and closing billions of times over a lifetime. The non-linear, viscoelastic, and anisotropic mechanical properties of valve tissue enable the efficient flow of blood through the heart for delivery to the rest of the body. But heart valves can become diseased, resulting in disorganized ECM and mechanical dysfunctions. Tissue engineered replacement valves must match the mechanical properties of native valves to replicate function and appropriately guide the behavior of cells seeded in the scaffold. We are using a composite scaffold of electrospun biodegradable polyurethane (BPUR) and poly(ethylene glycol) (PEG) hydrogel to mimic the heterogeneous valve ECM and mechanical properties.
Materials and Methods: PEG was functionalized with an enzymatically degradable peptide sequence, GGGPQGIWGQGK (PQ), to make PEG-PQ-PEG diacrylate. RGDS was conjugated to PEG for a cell adhesion ligand. Poly(ether ester urethane) urea with 50% hard segment was electrospun and collected on a rotating mandrel to produce anisotropic mechanical behavior. The electrospun mat was encapsulated inside PEG-PQ-PEG hydrogel with 2 mM PEG-RGDS and 15x106 valve interstitial cells (VICs)/ml. Mechanical properties of the scaffold were measured in uniaxial tension, and immunohistochemistry demonstrated phenotype of encapsulated cells.
Results and Discussion: The synthetic BPUR/PEG-PQ-PEG scaffold has many of the mechanical properties present in native valve tissue. The fibrous nature of the electrospun BPUR mimics ECM components collagen and elastin and the fiber alignment replicates the anisotropic behavior of valve tissue. Most synthetic materials exhibit a linear stress-strain response, which is different from the non-linear stress-strain curve of native valves (Figure 1A), especially in the 10-30% physiological strain range.
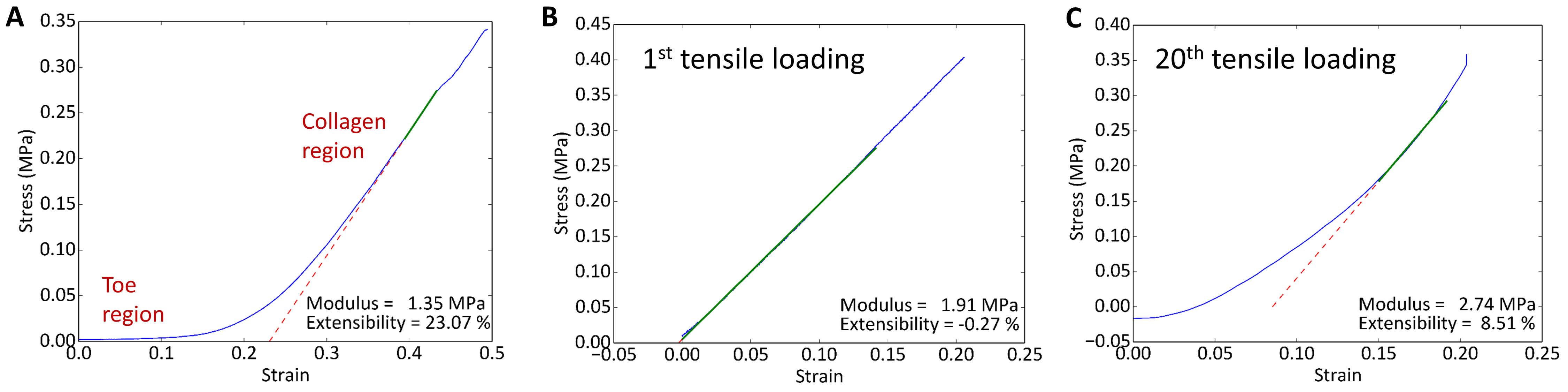
Figure 1: (A) Uniaxial tensile testing of aortic valve tissue in radial direction. Stress vs. strain plot is nonlinear, with low-modulus toe region followed by transition to stiffer collagen region; (B) 1st and (C) 20th cyclic tensile loading of BPUR/PEG-PQ-PEG composite scaffold
Bilinear Preconditioning the BPUR in tension altered the microphase morphology such that a bilinear stress-strain response was observed upon subsequent tensile loading of the composite scaffold (Figure 1B-C shows the 1st and 20th cycles). The PEG hydrogel component was a bioactive cell carrier in which encapsulated VICs showed healthy phenotype in 3D.

Figure 2: Cross-section of top side of composite scaffold. VICs encapsulated in PEG-PQ-PEG hydrogel, which is between dashed lines; BPUR is UV auto-fluorescent (blue) in lower right corner
The cells sensed the stiffness and alignment of the electrospun fibers, without strong αSMA expression which would have been indicative of a diseased state. The next step will be to assess scaffold remodeling with cell secreted ECM after long term culture under cyclic tension.
Conclusion: The composite BPUR/PEG-PEG-PQ scaffold is a step towards a tissue engineered valve that mimics the structural and functional heterogeneity of native valves.