Introduction: Musculoskeletal tissue loss or damage resulting from trauma, surgery, or disease presents a significant medical challenge. Stem cell-based strategies by using autologous mesenchymal stem cells (MSCs) have great potential in regenerative engineering. Hydrogels composed of polymer networks swollen in water provide an attractive three-dimensional (3D) supportive environment to induce desirable cellular responses for regeneration of musculoskeletal tissues. The regenerative efficacy is greatly dependent on precise control and optimization of cell-material interactions. In the present study, we aim to develop a novel composite hydrogel as a bioengineered cellular niche for MSCs through combination of thiolated hyaluronic acid (HA) and chondroitin sulfate (CS) crosslinked with polyethylene glycol (PEG). The combination of HA and CS offers a biomimetic microenvironment found in cartilage whereas the selection of PEG as a crosslinker is based on its established biocompatibility and chemical versatility. It is hypothesized that the composite hydrogels will support MSC cell culture, and cell-hydrogel interactions can be effectively modulated by varying the properties of hydrogels.
Materials and Methods: Hyaluronic acid sodium salt and chondroitin sulfate sodium salt were purchased from Carbosynth Limited. Poly (ethylene glycol) (PEG) diacrylate (Mw700, 3400, and 8000) were purchased from Alfa Aesar. Thiolated HA and thiolated CS were synthesized by coupling HA or CS with cysteamine dihydrochloride using 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-Hydroxysuccinimide(NHS) as catalysts, and then were reduced by dithiothreitol. Polymer molecular structure was confirmed by nuclear magnetic resonance (NMR) spectroscopy. Rheological experiments were carried out with a TA Discovery Series Hybrid Rheometer (DHR)-3 using parallel plate (20 mm diameter, 0°) configuration at 37 °C in the oscillatory mode. In vitro studies were performed to examine cell-hydrogel interactions using human MSCs (hMSCs). hMSCs were encapsulated in hydrogels and cultured in growth media. Cell viability and growth was monitored by a live/dead assay. The dynamic monitoring of focal adhesion kinase (FAK) activity was conducted to examine cell-hydrogel interactions using fluorescence lifetime imaging microscopy (FLIM) with FAK phosphorylation biosensors[1].
Results and Discussion: The interpenetrating HA-CS-PEG biomimic hydrogels were fabricated via thiol-ene click chemistry (Fig. 1A). New resonances in proton NMR spectroscopy (1H NMR) of HA-SH appeared at δ 2.25 and δ 2.55, corresponding to the two side chain methylenes (-CH2CH2SH). The formation of hydrogel was conformed using the vial tilting method (Fig. 1B). Extensive rheology testing showed that increased molecular weight of the cross-linking agent (PEG) was able to increase the hydrogel stiffness (Fig. 1C). (p<0.05).
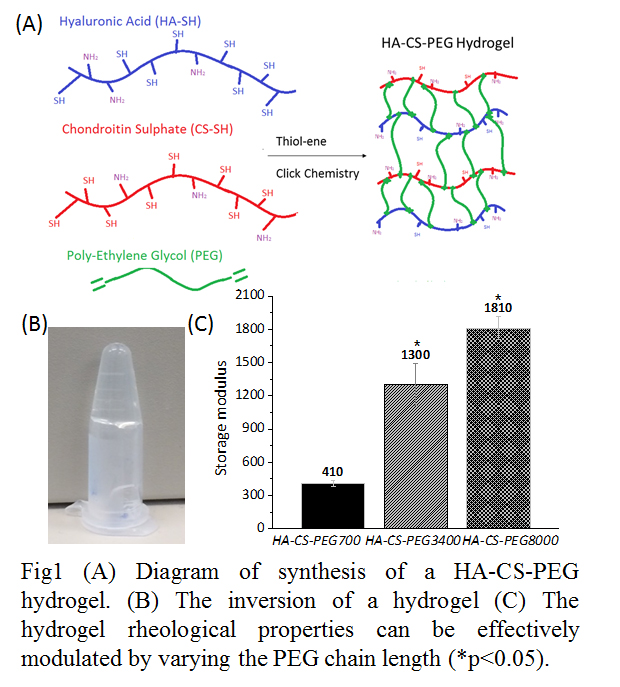
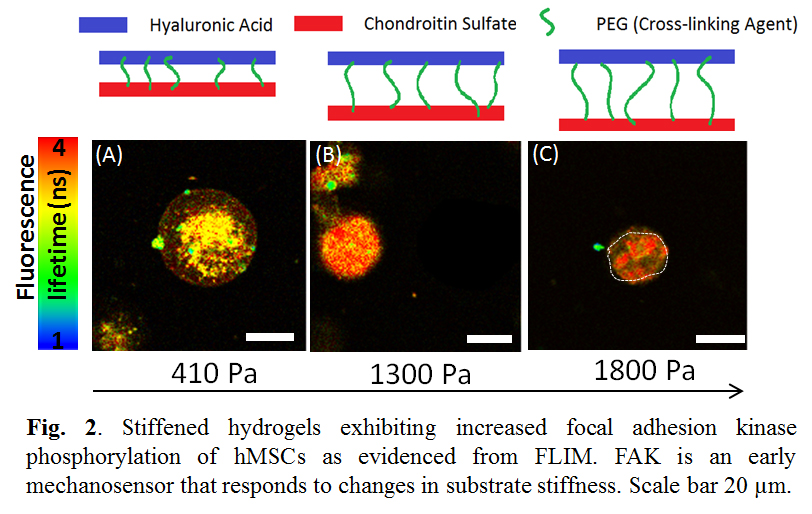
hMSCs with biosensors were encapsulated in HA-CS-PEG 700 (Fig.2A), HA-CS-PEG 3400 (Fig. 2B), and HA-CS-PEG 8000 (Fig. 2C) hydrogels, respectively. The fluorescence lifetime of the biosensors within the hydrogel increased as hydrogel stiffness increased, indicating FAK increased with increased stiffness. The cell size decreased as hydrogel stiffness increased as well.
Confocal images demonstrated that the composite hydrogels supported the cell growth with high viability during culture. Cells displayed round cell morphologies similar to those of chondrocytes in cartilage (Fig. 3A and 3B).

Conclusion: A novel composite hydrogel consisting of thiolated HA and CS cross-linked with PEG was developed through thiol-ene click chemistry. Variations in the cross-linking allowed for modulating physical properties of hydrogels and cell-hydrogel interactions (e.g. FAK phosphorylation). In vitro studies with hMSCs demonstrated the ability of the hydrogel to support 3D cell encapsulation with high viability. These experiments demonstrated the promise of fine-tuning properties of novel composite hydrogels for achieving optimal cellular responses, and paved the way for further development of regenerative technologies for treatment
of musculoskeletal injuries.
Showalter Trust; Purdue Research Foundation; Purdue Start-up Package
References:
[1] Damayanti, N. P., Parker, L. L. and Irudayaraj, J. M. K., “Fluorescence Lifetime Imaging of Biosensor Peptide Phosphorylation in Single Live Cells,” Angew. Chem. Int. Ed., 2013, 52: 3931–3934.