Introduction: Hydrogels derived from extracellular matrix (ECM) have been shown to mediate inflammation and promote a constructive tissue remodeling response. However, well-accepted terminal sterilization methods (i.e., gamma irradiation, electron beam irradiation, ethylene oxide (EtO) exposure) inhibit formation of an ECM hydrogel at physiologic conditions. It is necessary to identify a sterilization method conducive to ECM hydrogel formation prior to clinical translation. Supercritical CO2 (scCO2) sterilization, a relatively novel method of sterilization, was tested for its potential to sterilize and allow hydrogel formation. Preliminary work with porcine urinary bladder matrix (UBM) indicated that exposure to ethylene oxide and 30-kGy irradiation had detrimental effects on ECM hydrogel formation, and that scCO2 sterilization of lyophilized pre-gel was the only method to permit gel formation. The objective of the present study was to characterize the biologic and viscoelastic properties of sterilized lyophilized ECM hydrogels derived from various source tissues
Materials and Methods: Four tissues were decellularized for ECM hydrogel production: urinary bladder (UBM), small intestinal submucosa (SIS), liver (lECM) and bone (bECM). Following decellularization, tissues were digested with 1 mg/mL pepsin in 0.01N HCl at an ECM concentration of 10 mg/mL for 48 hours to produce pre-gel solutions which were lyophilized and subjected to scCO2 sterilization or 30-kGy gamma irradiation. Dried samples were then reconstituted in deionized water at 4°C overnight and pH neutralized and salt balanced. THP-1 human monocytes (ATCC TIB-202) were plated at 500,000 cells per well with 320 nM phorbol 12-myristate 13-acetate (PMA) for 24 hours to induce differentiation into macrophages. After 48 hours rest macrophages were treated with ECM hydrogels for 48 hours. Pre and post treatments with lipopolysaccharide (LPS, 100 ng/ml) were also conducted for two hours. Culture supernatants were centrifuged to pellet the cells and supernatants frozen. Biologic activity of the sterilized ECM hydrogels was assessed via TNFα, IL1-RA and PGE2 secretion with ELISAs. Rheological testing was performed with an AR 2000 rheometer to determine the storage (G’) and loss (G’’) moduli. Rheologic properties were recorded while raising temperature from 10°C to 37°C over the course of 1 minute then retaining these conditions for 1 hour.
Results and Discussion: There was no significant difference in the cytokines secreted by macrophages treated with sterilized and non-sterilized ECM hydrogels (Figure 1).
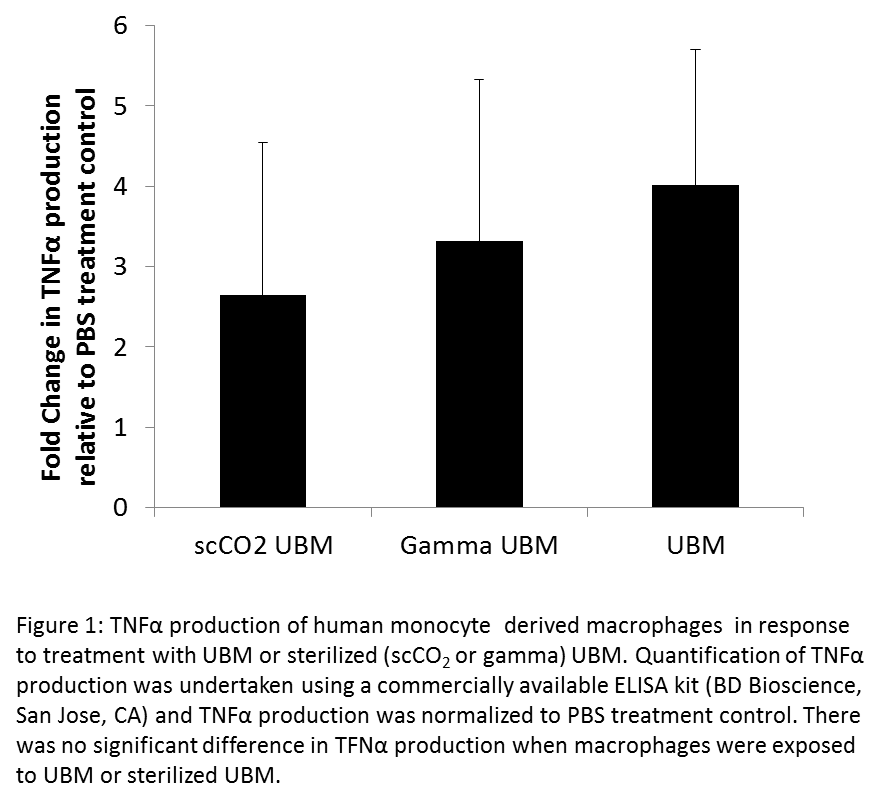
However, macrophages exposed to LPS prior to treatment with ECM hydrogels showed increased levels of TNFα secretion with gamma sterilized hydrogels compared to scCO2 sterilized hydrogels (Figure 2).
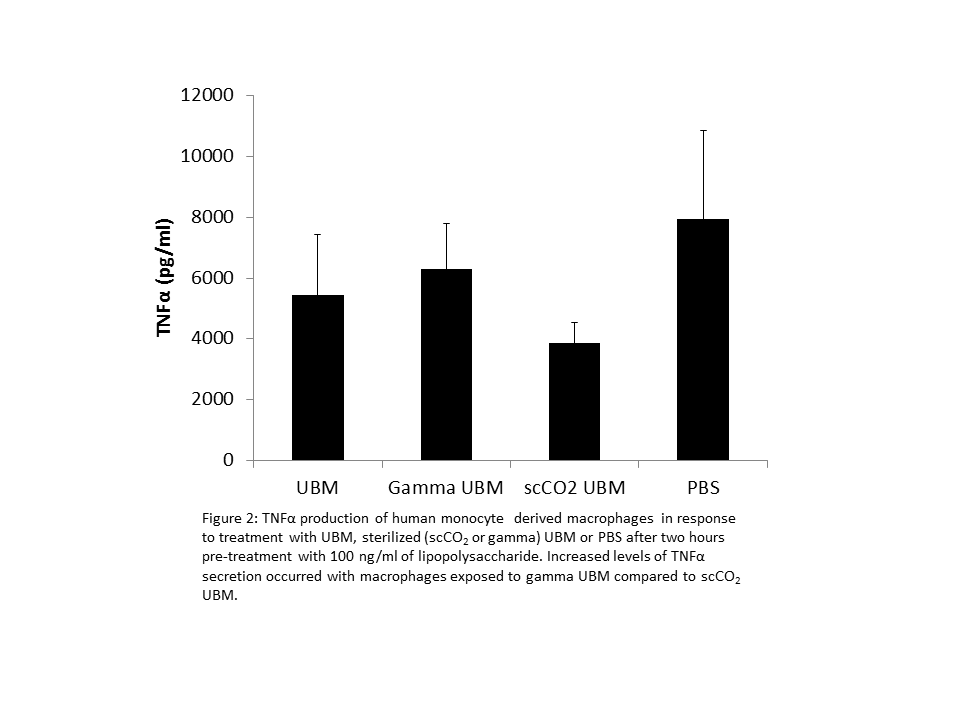
Additionally, ECM exposed to high dose (30 kGy) gamma irradiation did not form a gel, displaying liquid-like storage moduli. Rheological properties were not affected by scCO2 sterilization with the scCO2-sterilized ECM hydrogels showing equivalent stiffness values to non-sterilized control samples.
Conclusions: The choice of sterilization method dramatically affects the viscoelastic and biologic properties of ECM hydrogels. Sterilization with scCO2 permits the formation of a hydrogel whereas sterilization by any other method did not allow subsequent hydrogel formation. The immunomodulatory properties, a hallmark of ECM hydrogels, were also altered by sterilization. Only scCO2 sterilized hydrogels were capable of mitigating the inflammatory cascade prompted by treatment with LPS.
LJW is funded by an International Outgoing Fellowship (Marie Curie Actions) under the European Union's Seventh Framework Programme (FP7/2007-2013).